
Determine the number of unpaired electrons in each atom: K, Ca, Sc, Ti, V, Cr, Mn.
(a)
Interpretation:
The number of unpaired electrons in the given atoms should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.102QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
All the electrons are placed in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule
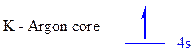
The one electron of
The unpaired electrons are present in
(b)
Interpretation:
The number of unpaired electrons in the given atoms should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.102QP
There is no unpaired electron in
Explanation of Solution
The noble gas core for
All the electrons are placed in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule
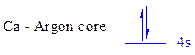
The two electrons of
There is no unpaired electron present in
(c)
Interpretation:
The number of unpaired electrons in the given atoms should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.102QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
All the electrons are placed in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
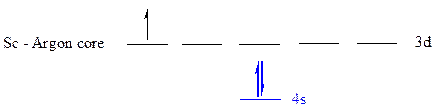
The three electrons of
The unpaired electrons are present in
(d)
Interpretation:
The number of unpaired electrons in the given atoms should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.102QP
The number of unpaired electrons in
Explanation of Solution
The noble gas core for
All the electrons are placed in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
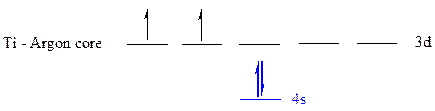
The four electrons of
The unpaired electrons are present in
(e)
Interpretation:
The number of unpaired electrons in the given atoms should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.102QP
The number of unpaired electrons in
Explanation of Solution
The noble gas core for
All the electrons are placed in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule
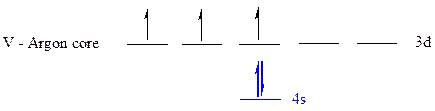
The five electrons of
The unpaired electrons are present in
(f)
Interpretation:
The number of unpaired electrons in the given atoms should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.102QP
The number of unpaired electrons in
Explanation of Solution
The noble gas core for
All the electrons are placed in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
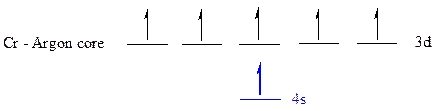
The six electrons of
The unpaired electrons are present in
(g)
Interpretation:
The number of unpaired electrons in the given atoms should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.102QP
The number of unpaired electrons in
Explanation of Solution
The noble gas core for
All the electrons are placed in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
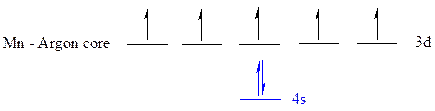
The seven electrons of
The unpaired electrons are present in
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry: Atoms First
- What is the physical significance of the value of 2 at a particular point in an atomic orbital?arrow_forwardThe changes in electron affinity as one goes down a group in the periodic table are not nearly as large as the variations in ionization energies. Why?arrow_forward6.2 Unlike XRF, AAS cannot be used for nondestructive testing. Explain why not.arrow_forward
- Cesium was discovered in natural mineral waters in 1860 by R. W. Bunsen and G. R. Kirchhoff, using the spectroscope they invented in 1859. The name came from the Latin caesius ("sky blue") because of the prominent blue line observed for this element at 455.5 nm. Calculate the frequency and energy of a photon of this light.arrow_forwardGive the possible values of a. the principal quantum number, b. the angular momentum quantum number, c. the magnetic quantum number, and d. the spin quantum number.arrow_forwardTitanium metal and Cr2+ have the same number of electrons. However, the electron configuration of Ti is [Ar] 4s23d2, but that of Cr2+ is [Ar] 3d4. Explain.arrow_forward
- The electron affinities of the elements from aluminum to chlorine are 44, 120, 74, 200.4, and 384.7 kJ/mol, respectively. Rationalize the trend in these values.arrow_forwardThe first-row transition metals from chromium through zinc all have some biologic function in the human body. How many unpaired electrons are present in each of these first-row transition metals in the ground state?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





