
Concept explainers
(a)
Interpretation:
The IUPAC name and common name for the given thioether has to be assigned.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an
Root word represents the longest continuous carbon skeleton of the organic molecule.
IUPAC rules for naming thioether:
- ✓ The base name is found from the longest carbon chain present in thioether.
- ✓ The suffix –thio has to be added in order to obtain the alkylthio group name. For example, ethyl becomes as ethylthio, methyl becomes as methylthio etc.
- ✓ Alkylthio name has to be placed first with the number (carbon atom to which the alkykthio group is attached) followed by the base name.
Rules for assigning common names to thioether:
For obtaining common name for thioether, two rules are applicable, one for symmetrical ethers and one for unsymmetrical ethers.
- ✓ For unsymmetrical thioethers, the two hydrocarbon groups that is attached to the oxygen atom is arranged in an alphabetical order and the word sulfide is added. The words are separated by a space. These names have three words with space between them.
- ✓ For symmetrical ethers, prefix di- is used. Then the word sulfide is added with a space between the two words. These names have two words with space between them.
(a)
Answer to Problem 3.144EP
IUPAC name for the given compound is ethylthioethane and common name is diethyl sulfide.
Explanation of Solution
Given structure of compound is shown below,
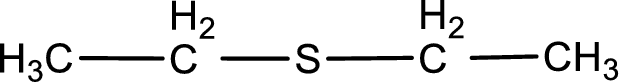
First step is to identify the longest carbon chain. In this case it is a two carbon chain. Hence, the base name is ethane.
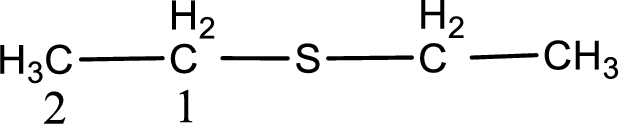
Next step is to identify the alkylthio group. In the given thioether, the alkylthio group is found to be ethylthio as it contains two carbon atoms.
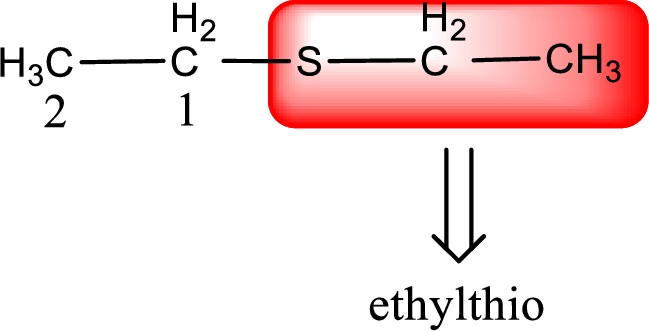
Alkylthio name is placed before the base name with the appropriate number that gives information about to which carbon atom the alkylthio group is attached. This gives the IUPAC name as ethylthioethane.
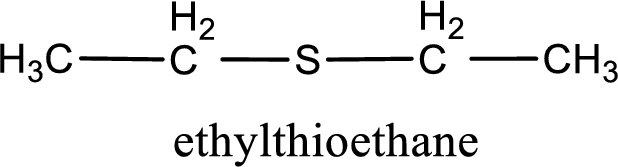
The IUPAC name of the given thioether is ethylthioethane.
To obtain common name the two hydrocarbon groups that are attached to the sulfur atom is named first. In the given structure, two ethyl groups are present. Therefore, prefix di- is added to the alkyl group followed by the word sulfide. Common name for the given thioether is diethyl sulfide.
IUPAC name and common name for the given thioether is assigned.
(b)
Interpretation:
The IUPAC name and common name for the given thioether has to be assigned.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc.
Root word represents the longest continuous carbon skeleton of the organic molecule.
IUPAC rules for naming thioether:
- ✓ The base name is found from the longest carbon chain present in thioether.
- ✓ The suffix –thio has to be added in order to obtain the alkylthio group name. For example, ethyl becomes as ethylthio, methyl becomes as methylthio etc.
- ✓ Alkylthio name has to be placed first with the number (carbon atom to which the alkykthio group is attached) followed by the base name.
Rules for assigning common names to thioether:
For obtaining common name for thioether, two rules are applicable, one for symmetrical ethers and one for unsymmetrical ethers.
- ✓ For unsymmetrical thioethers, the two hydrocarbon groups that is attached to the oxygen atom is arranged in an alphabetical order and the word sulfide is added. The words are separated by a space. These names have three words with space between them.
- ✓ For symmetrical ethers, prefix di- is used. Then the word sulfide is added with a space between the two words. These names have two words with space between them.
(b)
Answer to Problem 3.144EP
IUPAC name for the given compound is 2-ethylthiopropane and common name is ethyl isopropyl sulfide.
Explanation of Solution
Given structure of compound is shown below,
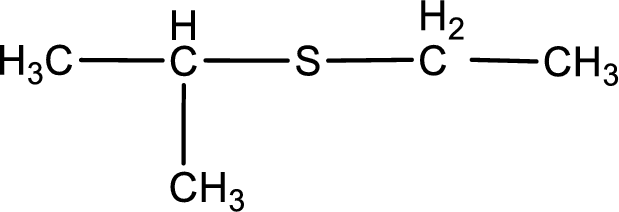
First step is to identify the longest carbon chain. In this case it is a three carbon chain. Hence, the base name is propane.
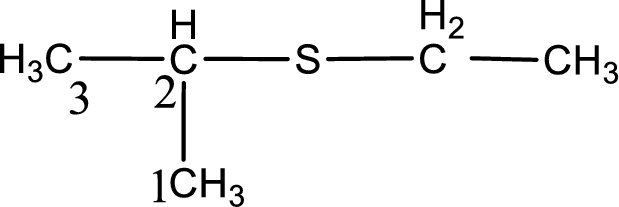
Next step is to identify the alkylthio group. In the given thioether, the alkylthio group is found to be ethylthio as it contains two carbon atoms. The point of attachment in the propane for ethylthio group is in the second carbon atom.
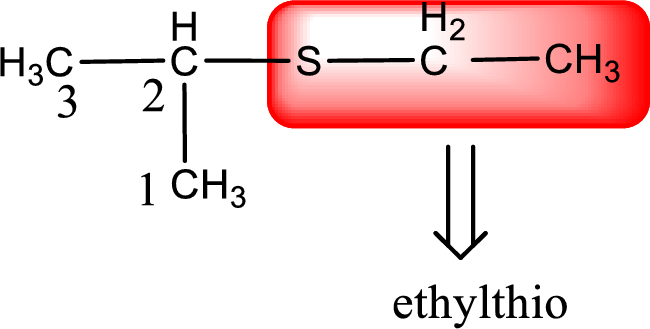
Alkylthio name is placed before the base name with the appropriate number that gives information about to which carbon atom the alkylthio group is attached. This gives the IUPAC name as 2-ethylthiopropane.
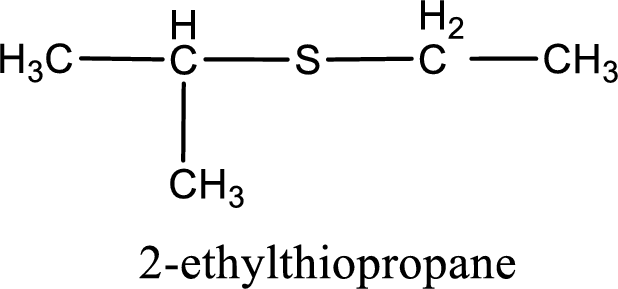
The IUPAC name of the given thioether is 2-ethylthiopropane.
To obtain common name the two hydrocarbon groups that are attached to the sulfur atom is named first. In the given structure, an ethyl and an isopropyl group is present. Arranging them in the alphabetical order and adding the word sulfide after them gives the common name for the given thioether. Common name for the given thioether is ethyl isopropyl sulfide.
IUPAC name and common name for the given thioether is assigned.
(c)
Interpretation:
The IUPAC name and common name for the given thioether has to be assigned.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc.
Root word represents the longest continuous carbon skeleton of the organic molecule.
IUPAC rules for naming thioether:
- ✓ The base name is found from the longest carbon chain present in thioether.
- ✓ The suffix –thio has to be added in order to obtain the alkylthio group name. For example, ethyl becomes as ethylthio, methyl becomes as methylthio etc.
- ✓ Alkylthio name has to be placed first with the number (carbon atom to which the alkykthio group is attached) followed by the base name.
Rules for assigning common names to thioether:
For obtaining common name for thioether, two rules are applicable, one for symmetrical ethers and one for unsymmetrical ethers.
- ✓ For unsymmetrical thioethers, the two hydrocarbon groups that is attached to the oxygen atom is arranged in an alphabetical order and the word sulfide is added. The words are separated by a space. These names have three words with space between them.
- ✓ For symmetrical ethers, prefix di- is used. Then the word sulfide is added with a space between the two words. These names have two words with space between them.
(c)
Answer to Problem 3.144EP
IUPAC name for the given compound is methylthiocyclopentane and common name is cyclopentyl methyl sulfide.
Explanation of Solution
Given structure of compound is shown below,
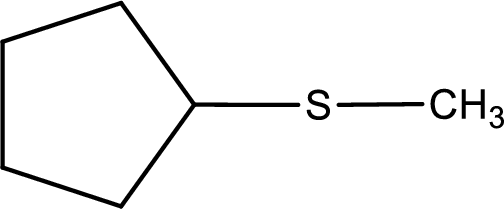
First step is to identify the longest carbon chain. In this case it is a five carbon cyclic chain that is saturated. Hence, the base name is cyclopentane.
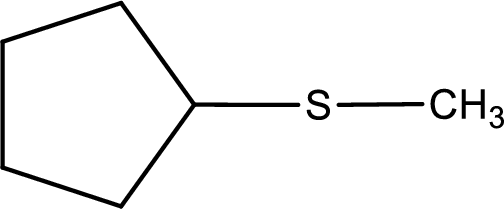
Next step is to identify the alkylthio group. In the given thioether, the alkylthio group is found to be methylthio as it contains only one carbon atom.
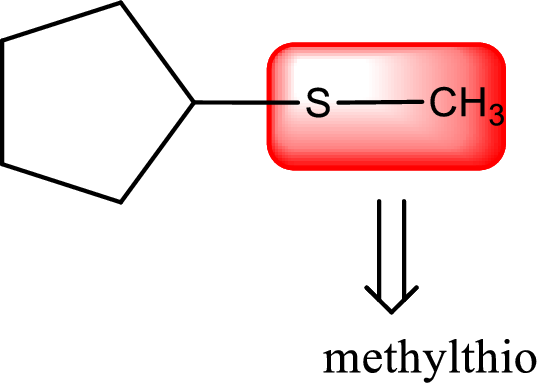
Alkylthio name is placed before the base name with the appropriate number that gives information about to which carbon atom the alkylthio group is attached. This gives the IUPAC name as methylthiocyclopentane.
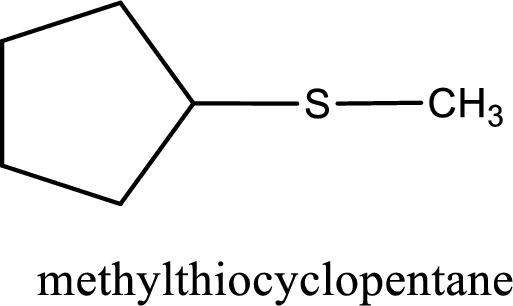
The IUPAC name of the given thioether is methylthiocyclopentane.
To obtain common name the two hydrocarbon groups that are attached to the sulfur atom is named first. In the given structure, a methyl and a cyclopentyl group is present. Arranging them in the alphabetical order and adding the word sulfide after them gives the common name for the given thioether. Common name for the given thioether is cyclopentyl methyl sulfide.
IUPAC name and common name for the given thioether is assigned.
(d)
Interpretation:
The IUPAC name and common name for the given thioether has to be assigned.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc.
Root word represents the longest continuous carbon skeleton of the organic molecule.
IUPAC rules for naming thioether:
- ✓ The base name is found from the longest carbon chain present in thioether.
- ✓ The suffix –thio has to be added in order to obtain the alkylthio group name. For example, ethyl becomes as ethylthio, methyl becomes as methylthio etc.
- ✓ Alkylthio name has to be placed first with the number (carbon atom to which the alkykthio group is attached) followed by the base name.
Rules for assigning common names to thioether:
For obtaining common name for thioether, two rules are applicable, one for symmetrical ethers and one for unsymmetrical ethers.
- ✓ For unsymmetrical thioethers, the two hydrocarbon groups that is attached to the oxygen atom is arranged in an alphabetical order and the word sulfide is added. The words are separated by a space. These names have three words with space between them.
- ✓ For symmetrical ethers, prefix di- is used. Then the word sulfide is added with a space between the two words. These names have two words with space between them.
(d)
Answer to Problem 3.144EP
IUPAC name for the given compound is 3-(ethylthio)-1-propene and common name is allyl ethyl sulfide.
Explanation of Solution
Given structure of compound is shown below,
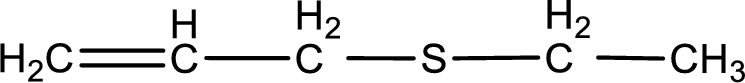
First step is to identify the longest carbon chain. In this case it is a three carbon chain with a double bond. Hence, the base name is propene.
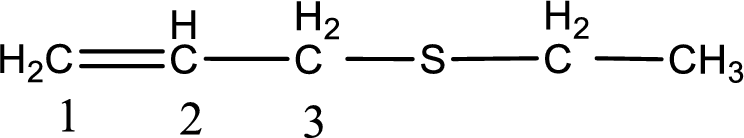
Next step is to identify the alkylthio group. In the given thioether, the alkylthio group is found to be ethylthio as it contains two carbon atoms.
Alkylthio name is placed before the base name with the appropriate number that gives information about to which carbon atom the alkylthio group is attached. This gives the IUPAC name as 3-(ethylthio)-1-propene.
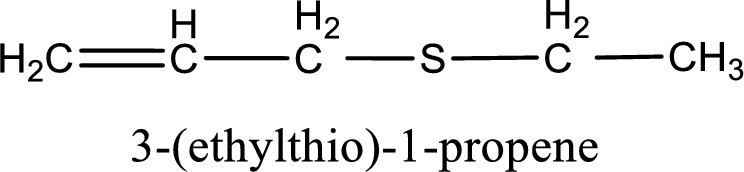
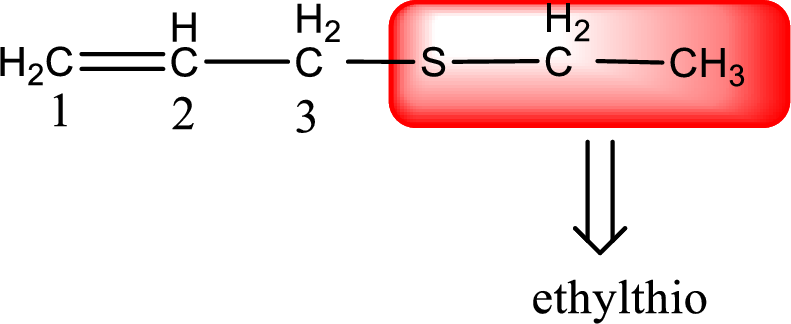
The IUPAC name of the given thioether is 3-(ethylthio)-1-propene.
To obtain common name the two hydrocarbon groups that are attached to the sulfur atom is named first. In the given structure, an ethyl and an allyl group is present. Arranging them in the alphabetical order and adding the word sulfide after them gives the common name for the given thioether. Common name for the given thioether is allyl ethyl sulfide.
IUPAC name and common name for the given thioether is assigned.
Want to see more full solutions like this?
Chapter 3 Solutions
Organic And Biological Chemistry
- Several important alcohols are well known by common names. Give a common name for each of the following: a. CH3OH b. c. CH3CH2OH d. e.arrow_forwardGive both the IUPAC name and the common name for each alcohol.(a) CH3CH2CH(OH)CH3arrow_forwardDraw the structure of each of the following molecules. (a) pentanoyl chloride; (b) 4-(2-methylpropyl)heptanedioyl chloride;(c) (S)-5-phenyloctanoyl chloridearrow_forward
- Give an IUPAC and common name for each of the following naturally occurring carboxylic acids: (a) CH3CH(OH)CO2H (lactic acid); (b) HOCH2CH2C(OH)(CH3)CH2CO2H (mevalonic acid).arrow_forwardDraw the product resulting from mild oxidation of (a) 2-butanol; (b) 2-methylpropanal; (c) cyclopentanol.arrow_forwardDraw the following compounds based on their IUPAC Names. 1. 5-cycloheptyl-2-isopropyl-5-oxopentanal 2. 4-hydroxybut-2-enoyl chloridearrow_forward
- Give the IUPAC or common name of each compound. (G- H)arrow_forwardGive the structure corresponding to each IUPAC name: a) 3-methyl-3-pentanol b) 4-methyl -2-pentanol c) 2,4 dimethyl -2-hexanol d) 1,3-propanediol e) 3,5-dimethylcyclohexanol f) 6,6-diethyl-4-nonanolarrow_forwardGive the IUPAC name and a common name for the following ether CH3-CH2-O-CH2-CH2-CH3 CH3-O-CH3arrow_forward
- The correct IUPAC name of the compound (see atatched picture) A.) 1-carbonyl-2-methyl-4-pentanol B.) 1-carbaldehyde-2-methyl-4-pentanol C.) 4-hydroxy-2-methylpentanal D.) 5-carbonyl-4-methyl-4-pentanol E.) 2-hydroxy-2-methyl-5-pentanalarrow_forwardGive a systematic (IUPAC) name for each diol. ) HO¬(CH2)8¬OHarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



