
(a)
Interpretation:
The symbol and name for the element contains only two
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their
Answer to Problem 3.28E
The name of the element that contains only two
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains only two
The name of the element that contains only two
(b)
Interpretation:
The symbol and name for the element contains an unpaired
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the element that contains an unpaired
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains an unpaired
The name of the element that contains an unpaired
(c)
Interpretation:
The symbol and name for the element contains two unpaired
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the elements that contain two unpaired
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains two unpaired
The element having electronic configuration
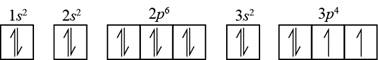
Figure 1
The element having electronic configuration
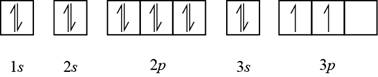
Figure 2
Hence, sulfur and silicon elements contain two unpaired
The name of the elements that contain two unpaired
(d)
Interpretation:
The symbol and name for the element contains three
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the element that contains three
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that three
The name of the element that contains three
(e)
Interpretation:
The symbol and name for the element contains three unpaired
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the element that contains three unpaired
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains three unpaired
The element having electronic configuration
![]()
Figure 3
The element having electronic configuration
![]()
Figure 4
Hence, vanadium and cobalt elements contain three unpaired
The name of the element that contains three unpaired
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- What is the maximum number of s orbitals found in a given electron shell? The maximum number of p orbitals? Of d orbitals? Of f orbitals?arrow_forwardWhich atom has the electron configuration 1s22s22p63s23p63d74s2?arrow_forwardGive the symbol of the element of lowest atomic number that has (a) an f subshell with 7 electrons. (b) twelve d electrons. (c) three 3p electrons. (d) a completed p subshell.arrow_forward
- Where are the most nonmetallic elements located on the periodic table? Why do these elements pull electrons from metallic elements so effectively during a reaction?arrow_forwardIdentify the two atoms with the same number of electrons in their outermost energy level. a. Na/K b. K/Ca c. Na/Mg d. Ca/Naarrow_forwardGive electron configurations according to the Bohr model for each of the following elements. Try to not use Figure 3.11, but instead determine the configuration based on your knowledge of the number of electrons in each atom and the maximum number of electrons in each Bohr orbit. Indicate which of these elements you expect to be the most reactive and the least reactive. a. B b. Si c. Ca d. F e. Ararrow_forward
- Which elements in a given period (horizontal row) of the periodic table lose electrons most easily? Why?arrow_forwardThe “Chemistry in Focus" segment The Chemistry of Bohrium discusses element 107. bohrium (Bh). What is the expected electron configuration of Bh?arrow_forwardWhich of the following statements is(are) true? a. The 2s orbital in the hydrogen atom is larger than the 3s orbital also in the hydrogen atom. b. The Bohr model of the hydrogen atom has been found to be incorrect. c. The hydrogen atom has quantized energy levels. d. An orbital is the same as a Bohr orbit. e. The third energy level has three sublevels, the s, p, and d sublevels.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





