
Interpretation:
The table has to be completed by filling in the formula of the ionic compound from the cations and anions.
Explanation of Solution
The cation is
Bromide
Hydroxide
Hydrogen carbonate
Sulfite
Phosphate
The cation is
Bromide
Hydroxide
Hydrogen carbonate
Sulfite
Phosphate
The cation is
Bromide
Hydroxide
Hydrogen carbonate
Sulfite
Phosphate
The figure below shows the complete table by filling in the formula of the ionic compound from the cations and anions is,
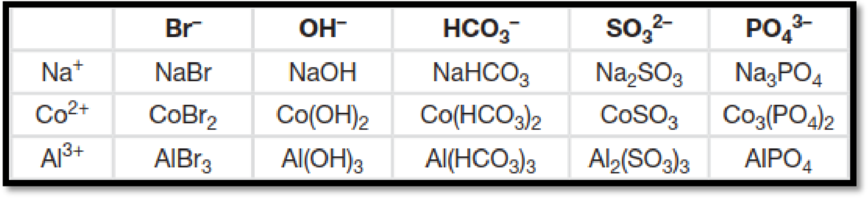
Figure 1
Want to see more full solutions like this?
Chapter 3 Solutions
Principles of General, Organic, Biological Chemistry
- Write formulas for the following binary ionic compounds: a. mercury(I)oxide b. lead(II)oxide c. platinum(IV)iodide d. copper(I)nitride e. cobalt(II)sulfidearrow_forwardFor each of the following compounds, state whether it is ionic or covalent. If it is ionic, write the symbols for the ions involved: NF3 BaO ( NH4)2CO3 Sr(H2 po4)2 IBr Na2Oarrow_forwardWrite chemical formulas for the following binary ionic compounds. a. Iron(III) oxide b. Iron(II) oxide c. Nickel(III) sulfide d. Copper(I) bromidearrow_forward
- Distinguish between the following terms. a. molecule versus ion b. covalent bonding versus ionic bonding c. molecule versus compound d. anion versus cationarrow_forwardDistinguish between the following terms. a. molecule versus ion b. covalent bonding versus ionic bonding c. molecule versus compound d. anion versus cationarrow_forwardWrite chemical formulas for the following binary ionic compounds. a. Gallium nitride b. Zinc chloride c. Magnesium sulfide d. Aluminum nitridearrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





