
Concept explainers
Identify the more stable stereoisomer in each of the following pairs, and give the reason for your choice:

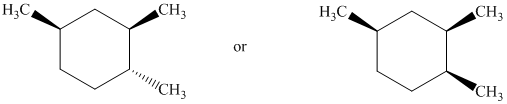
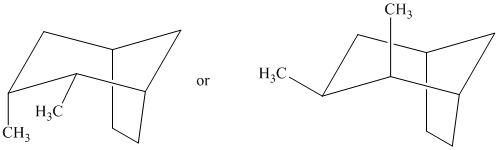
Interpretation:
The most stable stereoisomer in each of the given pairs is to be identified and the reason for it is to be explained.
Concept introduction:
The most stable conformation is the one that has the largest number of substituents in the equatorial orientation.
An axial substituent in the molecule is said to be crowded because of
Crowding causes increase in the potential energy of an isomer, which decreases its stability.
The basic chair conformation of cyclohexane is shown below:
Stereoisomers are isomers having the same constitution but differ in the arrangement of atoms in space. Cis-trans isomers are stereoisomers.
Two substituents are cis to each other if they are on the same side of the ring.
Two substituents are trans to each other if they are on the opposite side of the ring.
Answer to Problem 40P
Solution:
a)
In the cis isomer, the methyl substituent is in the axial orientation while in the trans isomer, the methyl substituent is in the equatorial orientation. The axial methyl group experiences
Thus, the trans isomer (B) is more stable than the cis isomer (A).
b)
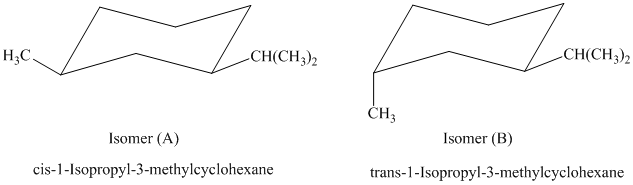
In isomer (A), the methyl substituent is in the equatorial orientation while in isomer (B), the methyl substituent is in the axial orientation. The axial methyl group in isomer (B) experiences
Thus, isomer (A) is more stable than isomer (B).
c)
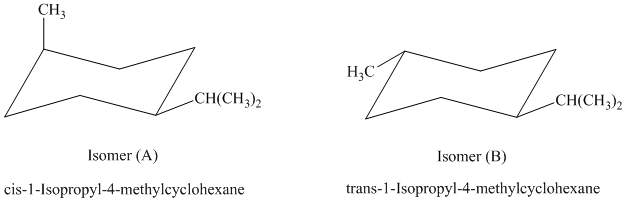
In isomer (A), the methyl substituent is in the axial orientation while in isomer (B), the methyl substituents is in the equatorial orientation. The axial methyl group in isomer (A) experiences
Thus, isomer (B) is more stable than isomer (A).
d)
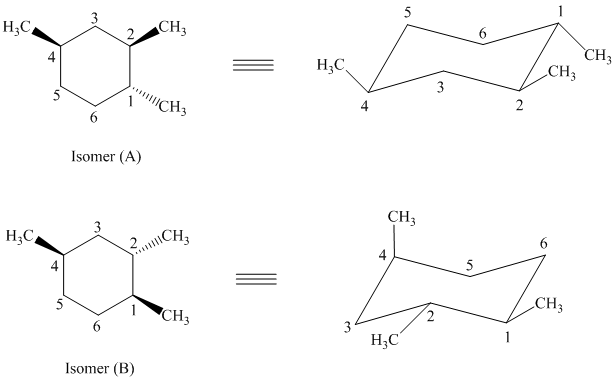
In isomer (A), all three methyl substituents are in an equatorial orientation while in isomer (B), one methyl substituent
Thus, isomer (A) is more stable than isomer (B).
e)
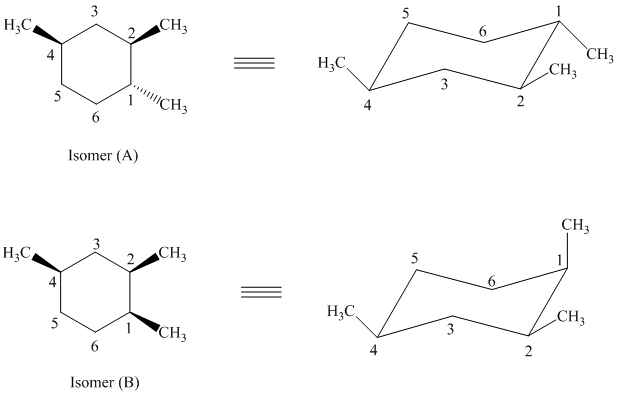
In isomer (A) all three methyl substituents are in an equatorial orientation while in isomer (B), one methyl substituent is in the axial orientation. The axial methyl group in isomer (B) experiences
Thus, isomer (A) is more stable than isomer (B).
f)
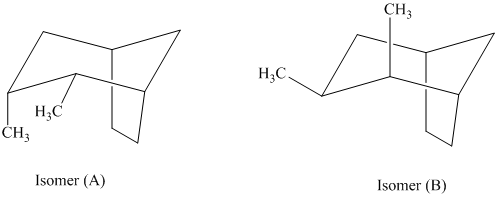
In isomer (A), the two methyl groups are on the same side as the cyclopentane ring and its hydrogen atoms. In isomer (B), the two methyl groups are on the opposite side of the cyclopentane ring and its hydrogen atoms. Isomer (A) is more crowded than isomer (B). Crowding increases the potential energy of the molecule and makes the molecule less stable.
Thus, isomer (B) is more stable than isomer (A).
Explanation of Solution
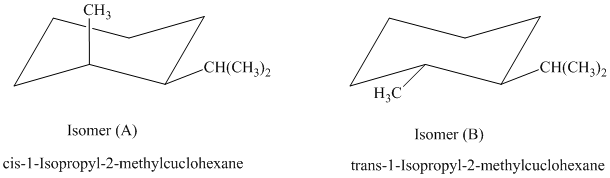
a) The given name of the compound shown below is
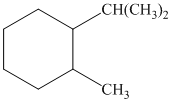
For cis and trans isomers of this compound, two substituents, an isopropyl and methyl groups at
The most stable cis and trans isomers for this compound are shown below:
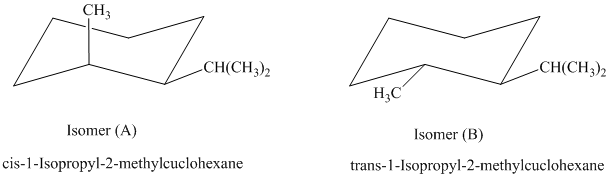
In isomer (A), the methyl substituent is in the axial orientation while in isomer (B), the methyl substituents is in the equatorial orientation. The axial methyl group experiences
Thus, isomer (B) is more stable than isomer (A).
b) The given name of the compound shown below is
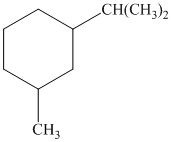
For cis and trans isomers of
The most stable cis and trans isomers for this compound are shown below:
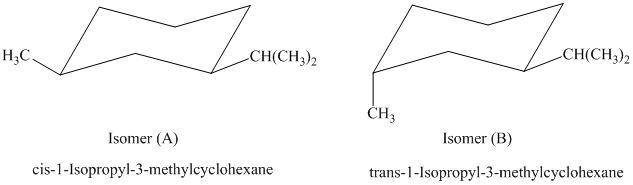
In isomer (A), the methyl substituent is in the equatorial orientation while in isomer (B), the methyl substituent is in the axial orientation. The axial methyl group in isomer (B) experiences
Thus, isomer (A) is more stable than isomer (B).
c) The given name of the compound shown below is
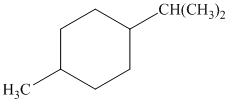
For cis and trans isomers of this compound, two substituents, an isopropyl and methyl groups at
The most stable cis and trans isomers for this compound are shown below:
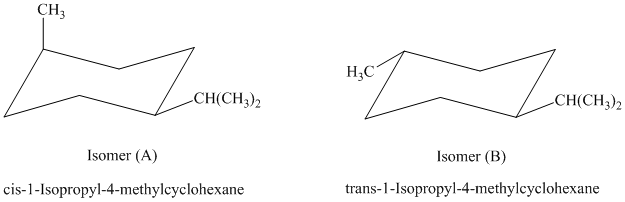
In isomer (A), the methyl substituent is in the axial orientation while in isomer (B), the methyl substituents is in the equatorial orientation. The axial methyl group in isomer (A) experiences
Thus, isomer (B) is more stable than isomer (A).
d) The given structures for two stereoisomers are as follows:
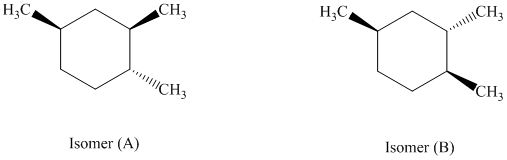
Both of the given isomers have three methyl groups attached to the cyclohexane. Their most stable conformations are shown below:
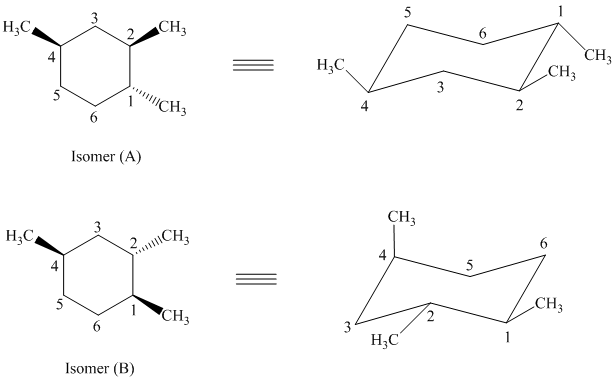
In isomer (A), all three methyl substituents are in an equatorial orientation while in isomer (B), two methyl substituenst at
Thus, isomer (A) is more stable than isomer (B).
e) The given structures for two stereoisomers are as follows:
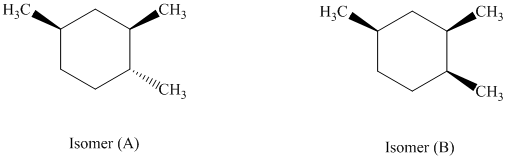
Both of the given isomers have three methyl groups attached to the cyclohexane. Their most stable conformations are shown below:
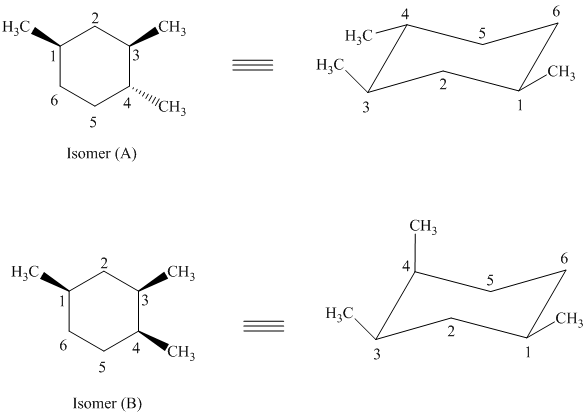
In isomer (A), all three methyl substituents are in an equatorial orientation while in isomer (B), two methyl substituents are in axial orientation. The axial methyl group in isomer (B) experiences
Thus, isomer (A) is more stable than isomer (B).
f) The given structures for two stereoisomers are as follows:
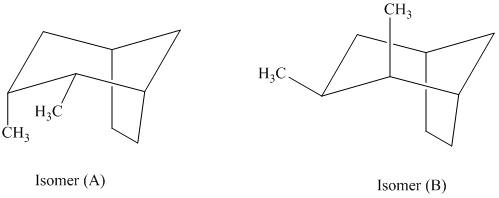
Both of the conformations represent two different cis conformations of the same compound. In isomer (A), the two methyl groups and hydrogen atoms on the cyclopentane ring are on the same side. In isomer (B), the two methyl groups and hydrogen atoms on the cyclopentane rings are on the opposite side. Isomer (A) is more crowded than isomer (B). Crowding increases potential energy of the molecule and makes the molecule less stable.
Thus, isomer (B) is more stable than isomer (A).
Want to see more full solutions like this?
Chapter 3 Solutions
Organic Chemistry - Standalone book
- Consider 1-bromo-2-methylpropane and draw the following. (a) The staggered conformation(s) of lowest energy (b) The staggered conformation(s) of highest energyarrow_forwardTell whether the following pairs of compounds are identical, constitutional isomers, stereoisomers, or unrelated. (a) cis-1, 3-Dibromocyclohexane and trans-1, 4-dibromocyclohexane (b) 2, 3-Dimethylhexane and 2, 3, 3-trimethy1pentanearrow_forwardIdentify each substituent in the following compound as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (green = Cl):arrow_forward
- Ketones react with alcohols to yield products called acetals. Why does the all-cis isomer of 4-tert-butyl-1,3-cyclohexanediol react readily with acetone and an acid catalyst to form an acetal, but other stereoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product acetal for each one.arrow_forwardDraw all possible stereoisomers for each of the following. Indicate those compounds for which no stereoisomers are possible. a. 1-bromo-2-chlorocyclohexane b. 2-bromo-4-methylpentane c. 1,2-dichlorocyclohexane d. 2-bromo-4-chloropentane e. 1-bromo-4-chlorocyclohexane f. 1,2-dimethylcyclopropane g. 4-bromo-2-pentene h. 3,3-dimethylpentane i. 1-bromo-2-chlorocyclobutane j. 1-bromo-3-chlorocyclobutanearrow_forwardWrite the two chair conformations of each of the following and in each part designate which conformation would be more stable. Why?a) trans-1-tert-butyl-3-methylcyclohexaneb) trans-1-tert-butyl-4-methylcyclohexanearrow_forward
- 1,2,3,4,5,6-Hexachlorocyclohexane shows cis,trans isomerism. At one time, a crude mixture of these isomers was sold as an insecticide. The insecticidal properties of the mixture arise from one isomer, known as lindane, which is cis-1,2,4,5-trans- 3,6-hexachlorocyclohexane. Q.) Draw a chair conformation for lindane and label which chlorine atoms are axial and which are equatorial.arrow_forwardDraw the most stable conformers of trans-1-Bromo-4-methylcyclohexane and cis-1-Bromo-4-methylcyclohexane. Which is more stable? Explain the reason for your answer.arrow_forwardDraw the two chair conformations of each compound, and label the substituents as axial and equatorial. In each case, determine which conformation is more stable. a) trans-1-ethyl-3-methylcyclohexanearrow_forward
- Determine the degree of unsaturation and then draw possible structures for noncyclic compounds with the following molecular formulas: a. C3H6 b. C3H4 c. C4H6arrow_forwardDraw the most stable conformation for each of the following substituted cyclohexanes. Show all conformation in Newman projections. Fill in hydrogens to indicate unsubstituted carbons.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

