
Interpretation: The shorthand structures of poly (vinyl chloride) and nylon
Concept introduction: The molecules which are obtained by the combination of similar units and possessing high molecular mass are known as
Answer to Problem 30.1P
The shorthand structures of poly (vinyl chloride) and nylon 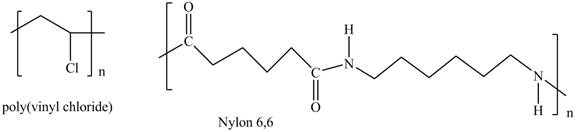
Explanation of Solution
The monomer required for the formation of poly (vinyl chloride) is vinyl chloride.
A shorthand structure of polymers represents the brackets that are placed around the repeating unit of the chain.
The shorthand structure of poly (vinyl chloride) and nylon
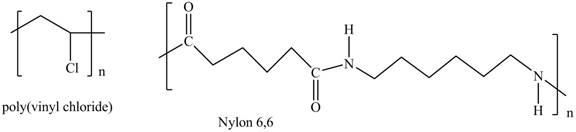
Figure 1
The shorthand structures of poly (vinyl chloride) and nylon
Want to see more full solutions like this?
Chapter 30 Solutions
ORGANIC CHEMISTRY CONNECT CODE
- Briefly Differentiate between Tg and Tm polymers.arrow_forwardTalk about polystyrene in general (detailed explanation), while writing references cited from itarrow_forwardHydrogen bonding between polyamide chains plays animportant role in determining the properties of a nylonsuch as nylon 6,6 (Table 12.6). Draw the structuralformulas for two adjacent chains of nylon 6,6 and showwhere hydrogen-bonding interactions could occur betweenthem.arrow_forward
- Give handwritten answer- Write three characteristic features for Zieglar Natta polymerization.arrow_forwardNovolac is the linear polymer which on heating with formaldehyde forms cross-linked bakelite. Write the structures of monomers and the polymer novolac.arrow_forwardwhat is the polyESTER version of nylon-6,10, and what monomers make the polyester?arrow_forward
- Identify the repeating unit in the final representation of Nylon 6,10arrow_forwardChemistry Write structure for oligopeptides “Boc-CPID-benzyl ester” and “Methy ester-CGLK-Boc”. Also show all the possible types of bonding between the two peptide chains.arrow_forwardKevlar and nomex are both polyamides. How do they differ? Write the structure of each.arrow_forward
- Draw the structure of leu-enkephalin, a pentapeptide that acts as an analgesic and opiate, and has the following sequence: Tyr–Gly–Gly–Phe–Leu. (The structure of a related peptide, met-enkephalin, appeared in Section 22.6B.)arrow_forwardWhat is the structural difference between HDP and LDP? How does the structure account for different behaviour and nature, hence the use of a polymer?arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





