
College Physics
1st Edition
ISBN: 9781938168000
Author: Paul Peter Urone, Roger Hinrichs
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30, Problem 31PE
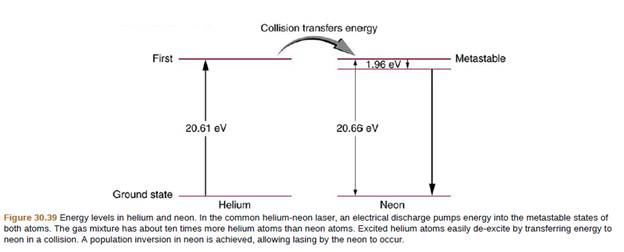
A helium-neon laser is pumped by electric discharge. What wavelength
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Barium has a work function of 2.48 eV. What is the maximum kinetic energy of electrons if the metal is illuminated by UV light of wavelength 375 nm? What is their speed?
A pulsed ruby laser emits light at 694.3 nm. For a 14.7-ps pulse containing 3.40 J of energy, find the following.
(a) the physical length of the pulse as it travels through space _________________mm(b) the number of photons in it__________________ photons(c) If the beam has a circular cross section 0.600 cm in diameter, find the number of photons per cubic millimeter. _______________photons/mm3
A sheet of metal is illuminated by photons with a wavelength of 325 nm and the emitted electrons are found to have a maximum kinetic energy of 1.25 eV. If the same metal is illuminated by 225 nm light, what will be the maximum speed of emitted electrons? Give your answer in km/s to 3 significant digits.
Chapter 30 Solutions
College Physics
Ch. 30 - Name three different types of evidence for the...Ch. 30 - Explain why patterns observed in the periodic...Ch. 30 - If atoms exist, why can't we see them with visible...Ch. 30 - What two pieces of evidence allowed the first...Ch. 30 - How do the allowed orbits for electrons in atoms...Ch. 30 - How do the allowed orbits for electrons in atoms...Ch. 30 - Explain how Bohr's rule for the quantization of...Ch. 30 - What is a hydrogen-like atom, and how are the...Ch. 30 - Explain why characteristic x rays are the most...Ch. 30 - Why does the energy of characteristic x rays...
Ch. 30 - Observers at a safe distance from atmospheric test...Ch. 30 - Lasers are used to burn and read CDs. Explain why...Ch. 30 - Crystal lattices can be examined with x rays but...Ch. 30 - CT scanners do not detect details smaller than...Ch. 30 - How do the allowed orbits for electrons in atoms...Ch. 30 - Atomic and molecular spectra are discrete. What...Ch. 30 - Hydrogen gas can only absorb EM radiation that has...Ch. 30 - Lasers are used to burn and read CDs. Explain why...Ch. 30 - The coating on the inside of fluorescent light...Ch. 30 - What is the difference between fluorescence and...Ch. 30 - How can you tell that a hologram is a true...Ch. 30 - How is the de Broglie wavelength of electrons...Ch. 30 - What is the Zeeman effect, and what type of...Ch. 30 - Define the quantum numbers n,l,ml,s, and ms.Ch. 30 - For a given value of n, what are the allowed...Ch. 30 - For a given value of l, what are the allowed...Ch. 30 - List all the possible values of s and msfor an...Ch. 30 - Identify the shell, subshell, and number of...Ch. 30 - Which of the following are not allowed? State...Ch. 30 - Using the given charge-to-mass ratios for...Ch. 30 - (a) Calculate the mass of a proton using the...Ch. 30 - If someone wanted to build a scale model of the...Ch. 30 - Rutherford found the size of the nucleus to be...Ch. 30 - In Millikan's oil-drop experiment, one looks at a...Ch. 30 - (a) An aspiring physicist wants to build a scale...Ch. 30 - By calculating its wavelength, show that the first...Ch. 30 - Find the wavelength of the third line in the Lyman...Ch. 30 - Look up the values of the quantities in...Ch. 30 - Verify that the ground state energy E0 is 13.6 eV...Ch. 30 - If a hydrogen atom has its electron in the n=4...Ch. 30 - A hydrogen atom in an excited state can be ionized...Ch. 30 - Find the radius of a hydrogen atom in the n=2...Ch. 30 - Show that (13.6eV)/hc=1.097107m=R (Rydberg's...Ch. 30 - What is the smallest-wavelength line in the Balmer...Ch. 30 - Show that the entire Paschen series is in the...Ch. 30 - Do the Balmer and Lyman series overlap? To answer...Ch. 30 - (a) Which line in the Balmer series is the first...Ch. 30 - A wavelength of 4.653 m is observed in a hydrogen...Ch. 30 - A singly ionized helium ion has only one electron...Ch. 30 - A beryllium ion with a single electron (denoted...Ch. 30 - Atoms can be ionized by thermal collisions, such...Ch. 30 - Verify Equations rn=n2ZaB and...Ch. 30 - The wavelength of the four Balmer series lines for...Ch. 30 - (a) What is the shortest-wavelength x-ray...Ch. 30 - A color television tube also generates some x rays...Ch. 30 - An x ray tube has an applied voltage of 100 kV....Ch. 30 - The maximum characteristic x-ray photon energy...Ch. 30 - What are the approximate energies of the K and K...Ch. 30 - Figure 30.39 shows the energy-level diagram for...Ch. 30 - A helium-neon laser is pumped by electric...Ch. 30 - Ruby lasers have chromium atoms doped in an...Ch. 30 - (a) What energy photons can pump chromium atoms in...Ch. 30 - Some of the most powerful lasers are based on the...Ch. 30 - If an atom has an electron in the n=5 state with...Ch. 30 - An atom has an electron with m1=2. What is the...Ch. 30 - What are the possible values of m1 for an electron...Ch. 30 - What, if any, constraints does a value of ml=1...Ch. 30 - (a) Calculate the magnitude of the angular...Ch. 30 - (a) What is the magnitude of the angular momentum...Ch. 30 - Repeat Exercise 30.40 for l=3.Ch. 30 - (a) How many angles can L make with the z-axis for...Ch. 30 - What angles can the spin S of an electron make...Ch. 30 - (a) How many electrons can be in the n=4 shell?...Ch. 30 - (a) What is the minimum value of 1 for a subshell...Ch. 30 - (a) If one subshell of an atom has 9 electrons in...Ch. 30 - (a) List all possible sets of quantum numbers...Ch. 30 - Which of the following spectroscopic notations are...Ch. 30 - Which of the following spectroscopic notations are...Ch. 30 - (a) Using the Pauli exclusion principle and the...Ch. 30 - Integrated Concepts Estimate the density of a...Ch. 30 - Integrated Concepts The electric and magnetic...Ch. 30 - (a) What is the distance between the slits of a...Ch. 30 - Integrated Concepts A galaxy moving away from the...Ch. 30 - Integrated Concepts Calculate the velocity of a...Ch. 30 - Integrated Concepts In a Millikan oil-drop...Ch. 30 - Integrated Concepts What double-slit separation...Ch. 30 - Integrated Concepts In a laboratory experiment...Ch. 30 - Integrated Concepts Find the value of l, the...Ch. 30 - Integrated Concepts Particles called muons exist...Ch. 30 - Integrated Concepts Calculate the minimum amount...Ch. 30 - Integrated Concepts A carbon dioxide laser used in...Ch. 30 - Integrated Concepts Suppose an MRI scanner uses...Ch. 30 - Integrated Concepts (a) An excimer laser used for...Ch. 30 - Integrated Concepts A neighboring galaxy rotates...Ch. 30 - Integrated Concepts A pulsar is a rapidly spinning...Ch. 30 - Integrated Concepts Prove that the velocity of...Ch. 30 - Unreasonable Results (a) What voltage must be...Ch. 30 - Unreasonable Results A student in a physics...Ch. 30 - Construct Your Own Problem The solar corona is so...Ch. 30 - Construct Your Own Problem Consider the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The precession angular velocity of a gyroscope is 1.0 rad/s. If the mass of the rotating disk is 0.4 kg and its...
University Physics Volume 1
TEST YOUR UNDERSTANDING OF SECTION 5.1 A traffic light of weight w hangs from two lightweight cables, one on ea...
University Physics with Modern Physics (14th Edition)
47. Figure 18.42 shows a system of four capacitors where the potential difference across ab is 50.0 V (a) Find ...
College Physics (10th Edition)
The pV-diagram of the Carnot cycle.
Sears And Zemansky's University Physics With Modern Physics
Mark the position of each of the labeled points at a later time when the wheel has completed one half of a turn...
Tutorials in Introductory Physics
1. An object is subject to two forces that do not point in opposite directions. Is it possible to choose their ...
College Physics: A Strategic Approach (4th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The metals sodium, iron, and molybdenum have work functions 25 eV, 3.9 eV, and 4.2 eV, respectively. Which of these metals will emit photoelectrons when illuminated with 400 nm light?arrow_forwardThe red light emitted by a ruby laser has a wavelength of 694.3 nm. What is the difference in energy between the initial state and filial state corresponding to the emission of the light?arrow_forwardA 600-nm light falls on a photoelectric surface and electrons with the maximum kinetic energy of 0.17 eV are emitted. Determine (a) the work function and (b) the cutoff frequency of the surface. (c) What is the stopping potential when the surface is illuminated with light of wavelength 400 nm?arrow_forward
- A 400-nm laser beam is projected onto a calcium electrode. The power of the laser beam is 2.00 mW and the work function of calcium is 2.31 eV. (a) How many photoelectrons per second are ejected? (b) What net power is carried away by photoelectrons?arrow_forwardThe velocity of a proton emerging from a Van de Graaff accelerator is 25.0% of the speed of light. (a) What is the proton's wavelength? (b) What is its kinetic energy, assuming it is nonrelativistic? (c) What was the equivalent voltage through which it was accelerated?arrow_forwardHow many photons are emitted during a 2.50-second burst of light from a 445 nm laser that operates at 0.400 watts?arrow_forward
- Monochromatic light from a laser shines onto a gold surface and produces photoelectrons with maximum kinetic energy KEmax = 7.5 eV. What is the wavelength of the laser light? Assume a work function of 5.10 eV for gold. answer in units of nm, rounded to 1 decimal place.arrow_forwardMonochromatic light from a laser shines onto a gold surface and produces photoelectrons with maximum kinetic energy KEmax = 9.7 eV. What is the wavelength of the laser light? Assume a work function of 5.10 eV for gold.Please give your answer in units of nm, rounded to 1 decimal place.arrow_forwardA metal surface is illuminated by light with a wavelength of 350 nm. The maximum kinetic energy of the emitted electrons is found to be 1.50 eV. What is the maximum electron kinetic energy if the same metal is illuminated by light with a wavelength of 250 nm?arrow_forward
- Light of wavelength 211 nm is shone on gold, which has a work function of 5.31 eV. What is the maximum kinetic energy (in eV) of the electrons emitted from the metal? Assume the light is traveling through a vacuum.arrow_forwardA laser emits a pulse of light with energy 5.0*10^3 J. How do we determine the number of photons in the pulse if the wavelength of light is 480 nm?arrow_forwardA helium-neon laser is pumped by electric discharge.What wavelength electromagnetic radiation would be needed to pump it? As shown for energy-level information.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill