
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30.SE, Problem 34AP
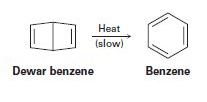
Bicyclohexadiene, also known as Dewar benzene, is extremely stable despite the fact that its rearrangement to benzene is energetically favored. Explain why the rearrangement is so slow.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
In 4+2 cycloaddition, ENDO stereochemistry is preferred because:
endo stereochemistry allows the diene to adopt s-cis conformation.
endo stereochemistry results in less steric hindrance.
the transition state leading to endo stereochemistry is higher energy.
it allows for additional π to π overlap between the diene and the dienophile.
Carbocations are carbons bearing a formal positive charge.
a. Carbocations can be stabilized by induction and hyperconjugation. Explain each of these forms of stabilization.
Show that the [4 + 4] cycloaddition of two butadiene molecules to give cycloocta1,5-diene is thermally forbidden but photochemically allowed
Chapter 30 Solutions
Organic Chemistry
Ch. 30.1 - Prob. 1PCh. 30.3 - Prob. 2PCh. 30.3 - Prob. 3PCh. 30.4 - Prob. 4PCh. 30.6 - What stereochemistry would you expect for the...Ch. 30.6 - Prob. 6PCh. 30.7 - Prob. 7PCh. 30.8 - Propose a mechanism to account for the fact that...Ch. 30.8 - When a 2, 6-disubstituted allyl phenyl ether is...Ch. 30.9 - Prob. 10P
Ch. 30.SE - Predict the product obtained when the following...Ch. 30.SE - Prob. 12VCCh. 30.SE - The following rearrangement of N-allyl-N,...Ch. 30.SE - Plastic photochromic sunglasses are based on the...Ch. 30.SE - Prob. 15MPCh. 30.SE - Prob. 16MPCh. 30.SE - Prob. 17MPCh. 30.SE - Prob. 18APCh. 30.SE - Prob. 19APCh. 30.SE - Prob. 20APCh. 30.SE - Prob. 21APCh. 30.SE - Prob. 22APCh. 30.SE - Prob. 23APCh. 30.SE - Prob. 24APCh. 30.SE - Prob. 25APCh. 30.SE - Prob. 26APCh. 30.SE - Prob. 27APCh. 30.SE - Prob. 28APCh. 30.SE - Propose a pericyclic mechanism to account for the...Ch. 30.SE - Prob. 30APCh. 30.SE - Prob. 31APCh. 30.SE - Prob. 32APCh. 30.SE - Prob. 33APCh. 30.SE - Bicyclohexadiene, also known as Dewar benzene, is...Ch. 30.SE - Prob. 35APCh. 30.SE - Prob. 36APCh. 30.SE - The 1H NMR spectrum of bullvalene at 100 C...Ch. 30.SE - Prob. 38APCh. 30.SE - Prob. 39APCh. 30.SE - Prob. 40APCh. 30.SE - In light of your answer to Problem 30-40, explain...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statements about cycloaddition reactions is not true? Cycloaddition reactions form a cyclic product with two new bonds. The course of the reaction is determined by the symmetry of the molecular orbitals of the products. Cycloaddition reactions are concerted. Cycloaddition reactions are stereospecific.arrow_forwardExplain the Carbocation Rearrangements ?arrow_forwardThe bicyclic alkene P can be prepared by thermal electrocyclic ringclosure from cyclodecadiene Q or by photochemical electrocyclic ringclosure from cyclodecadiene R. Draw the structures of Q and R, andindicate the stereochemistry of the process by which each reactionoccurs.arrow_forward
- Dewar benzene is a highly strained isomer of benzene. In spite of its thermodynamic instability, it is very stable kinetically. It will slowly rearrange to benzene, but only if heated to a very high temperature. Why is it kinetically stable?arrow_forwardWhich of the following rings is the most strained? cyclopropene; cyclopropane; cyclobutane; cyclopentane; cyclohexane after finding the most strained compound explain why and compare it to the other answer choices, explaining why they do not work as an answer.arrow_forwardA novel heterocyclic compound, M, contains nitrogen and sulfur atoms and exhibits interesting photochemical properties. When exposed to ultraviolet (UV) light at a specific wavelength, M undergoes a photoreaction resulting in two products: N and O. Product N is formed through a [2+2] cycloaddition involving the nitrogen atom in M, while product O results from a homolytic cleavage of a sulfur-sulfur bond in M. Given these reaction pathways, what is the most probable structure of M, and what are the likely structures of N and O? A. M is a thiazole derivative; N is a dimerized product through the nitrogen atoms, and O is a compound with two sulfur-centered radicals. B. M is a diazine derivative; N is a tetra-atomic cyclic compound, and O is a compound with two separate thiol groups. C. M is a thiadiazole derivative; N is a four-membered ring involving the nitrogen atom, and O results in two sulfur-centered radicals. D. M is a dithiolane derivative; N is a dimer involving the nitrogen…arrow_forward
- An electrophilic addition reaction of a conjugated diene that is thermodynamically-controlledarrow_forwardThis is a Diels-Alder reaction between ethylene and cis-1,3-butadiene. For each cycloaddition product, draw in all hydrogen atoms, and write the molecular formula below.arrow_forwardDiels–Alder reaction of a monosubstituted diene (such as CH2=CH–CH=CHOCH3) with a monosubstituted dienophile (such as CH2=CHCHO)gives a mixture of products, but the 1,2-disubstituted product oftenpredominates. Draw the resonance hybrid for each reactant, and use thecharge distribution of the hybrids to explain why the 1,2-disubstitutedproduct is the major product.arrow_forward
- A conjugated diene with an even number of double bonds undergoes conrotatory ring closure underthermal conditions.arrow_forwardEstimate the stabilization gained as a result of conjugation when 1,4-pentadiene is converted to trans-1,3-pentadiene. Note that the answer is not as simple as comparing the heats of hydrogenation of 1,4-pentadiene and trans-1,3pentadiene. Although the double bonds are moved from unconjugated to conjugated, the degree of substitution of one of the double bonds is also changed, in this case, from a monosubstituted double bond to a trans disubstituted double bond. To answer this question, you must separate the effect that is the result of conjugation from that caused by a change in the degree of substitution.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY