
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.12, Problem 12P
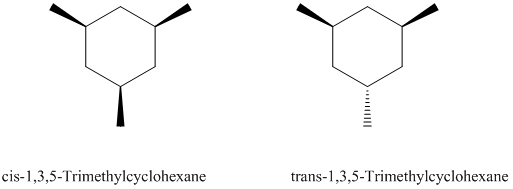
Based on what you know about disubstituted cyclohexanes, which of the following two stereoisomeric
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the systematic name for the 1,2,4-trichlorocyclohexane stereoisomer with the smallest heat of combustion?
can we convert this Trans-1-tert-butyl-3-methylcyclohexane into (the most stable version of)cis-1-tert-butyl-3-methylcyclohexane?what would cis-1-tert-butyl-3-methylcyclohexane be like?
If cyclopentane reacts with more than one equivalent of Cl2 at a high temperature, how many dichlorocyclo-pentanes would you expect to obtain as products?
Chapter 3 Solutions
Organic Chemistry - Standalone book
Ch. 3.1 - Identify the alkanes corresponding to each of the...Ch. 3.1 - Find the conformations in Figure 3.4 in which the...Ch. 3.2 - Sketch a potential energy diagram for rotation...Ch. 3.2 - Acetylcholine is a neurotransmitter in the central...Ch. 3.2 - Prob. 5PCh. 3.5 - The heats of combustion of ethylcyclopropane and...Ch. 3.8 - Prob. 7PCh. 3.10 - The following questions relate to a cyclohexane...Ch. 3.10 - Draw the most stable conformation of...Ch. 3.11 - Prob. 10P
Ch. 3.11 - Prob. 11PCh. 3.12 - Based on what you know about disubstituted...Ch. 3.12 - Write structural formulas for the most stable...Ch. 3.14 - Cubane (C4H8) is the common name of the polycyclic...Ch. 3.14 - Prob. 15PCh. 3.14 - Prob. 16PCh. 3.14 - Prob. 17PCh. 3.14 - Prob. 18PCh. 3.15 - Prob. 19PCh. 3 - Give the IUPAC names of each of the following: (a)...Ch. 3 - Draw Newman projections for the gauche and...Ch. 3 - Identify all atoms that are (a) anti and (b)...Ch. 3 - Prob. 23PCh. 3 - Prob. 24PCh. 3 - Prob. 25PCh. 3 - Prob. 26PCh. 3 - Prob. 27PCh. 3 - Prob. 28PCh. 3 - Oxidation of 4-tert-butylthiane proceeds according...Ch. 3 - The following are representations of two forms of...Ch. 3 - Draw (a) a Newman projection of the most stable...Ch. 3 - Write a structural formula for the most stable...Ch. 3 - Sight down the C-2-C-3 bond, and draw Newman...Ch. 3 - Prob. 34PCh. 3 - Sketch an approximate potential energy diagram for...Ch. 3 - Prob. 36PCh. 3 - Even though the methyl group occupies an...Ch. 3 - Which do you expect to be the more stable...Ch. 3 - Arrange the trimethylcyclohexane isomers shown in...Ch. 3 - Identify the more stable stereoisomer in each of...Ch. 3 - One stereoisomer of 1,1,3,5-tetramethylcyclohexane...Ch. 3 - One of the following two stereoisomers is...Ch. 3 - In each of the following groups of compounds,...Ch. 3 - The heats of combustion of the more and less...Ch. 3 - The measured dipole moment of ClCH2CH2Cl is 1.12D....Ch. 3 - Prob. 46PCh. 3 - Prob. 47PCh. 3 - Prob. 48DSPCh. 3 - Prob. 49DSPCh. 3 - Prob. 50DSPCh. 3 - Prob. 51DSPCh. 3 - Prob. 52DSPCh. 3 - Prob. 53DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw both cis- and trans-1,4-dimethylcyclohexane in their more stable chairconformations. (a) How many stereoisomers are there of cis-1,4-dimethylcyclohexane, and how many of trans-1,4-dimethylcyclohexane? (b) Are any of the structures chiral? (c) What are the stereochemical relationships among the various stereoisomers of 1,4-dimethylcyclohexane?arrow_forwardConsider 1-bromo-2-methylpropane and draw the following. (a) The staggered conformation(s) of lowest energy (b) The staggered conformation(s) of highest energyarrow_forwardKetones react with alcohols to yield products called acetals. Why does the all-cis isomer of 4-tert-butyl-1,3-cyclohexanediol react readily with acetone and an acid catalyst to form an acetal, but other stereoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product acetal for each one.arrow_forward
- What reagents in what order would you use to convert 1,2-dimethylcyclohexane to trans-1,2-dimethyl-1,2-dihydroxycyclohexane?arrow_forwardBetween trans-2-tert-butylcyclohexanol and cis-2-tert-butylcyclohexanol, which stereoisomer and configuration is the most stable? Why? Show where the steric strain occurrsarrow_forwardWhich of the following molecules is antiaromatic? A. cycloheptatrieneB. Cyclopentadienyl cationC. cyclopentadienyl anionD. cycloheptatrienyl cationE. silopentadienearrow_forward
- How many stereoisomers are formed from the reaction of 3-methylcyclohexene with NBS?arrow_forwardDraw all stereoisomers formed by monobromination of the cis andtrans isomers of 1,2-dimethylcyclohexane attached belowarrow_forward4-Chloro-2-pentene has a double bond that can have either the E or the Z configuration and a stereogenic center that can have either the R or the S configuration. How many stereoisomers arepossible altogether? Draw the structure of each, and group the pairs of enantiomers.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License