
Concept explainers
(a)
The linear atomic density of atom.
(a)
Answer to Problem 60AAP
The linear atomic density of atom is
Explanation of Solution
Draw the figure for the plane of
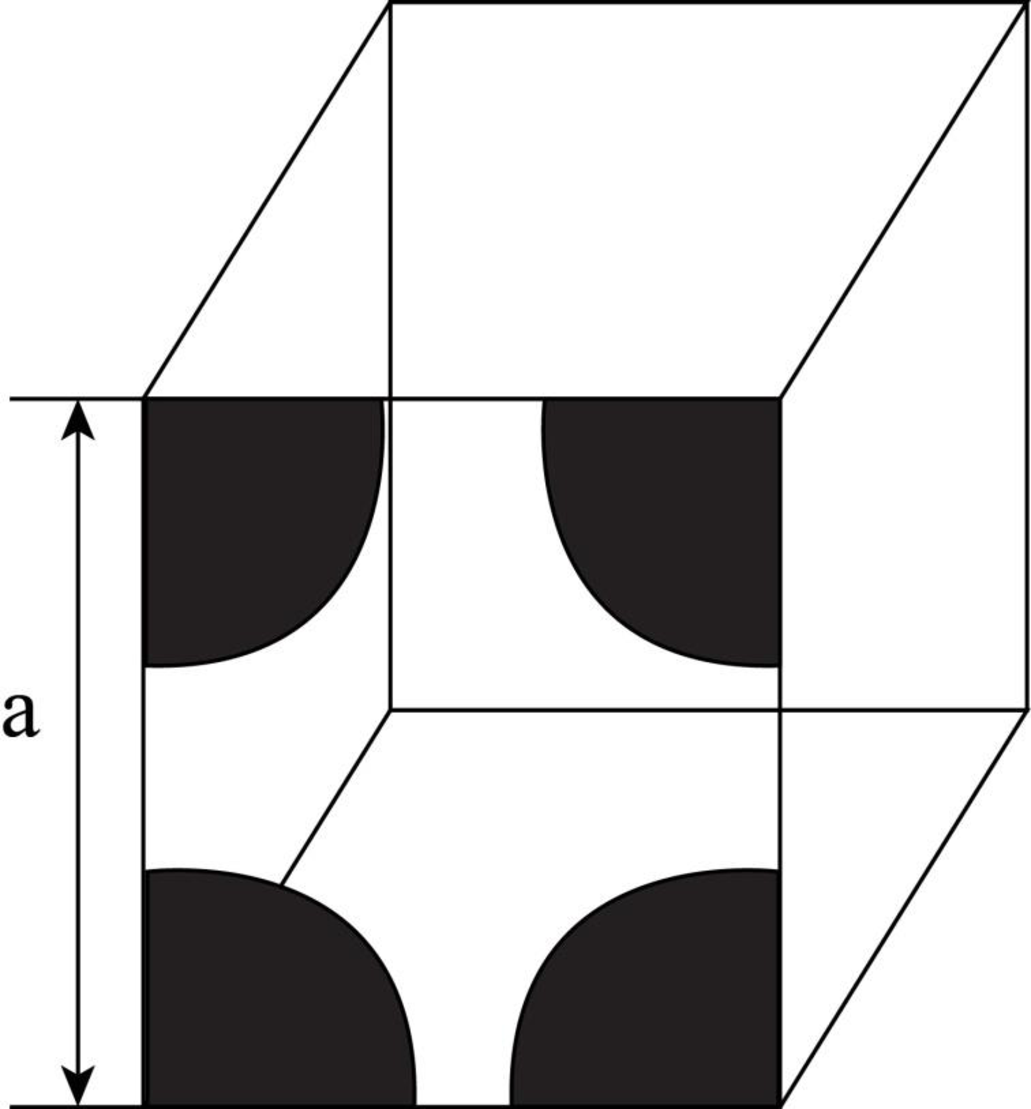
Figure-(1)
Write the expression for the planer density of the atom.
Here, the number of the atoms is
Conclusion:
Here, the number of atoms for
Substitute
Thus, the linear atomic density of atom is
(b)
The linear atomic density of atom.
(b)
Answer to Problem 60AAP
The linear atomic density of atom is
Explanation of Solution
Draw the figure for the plane of
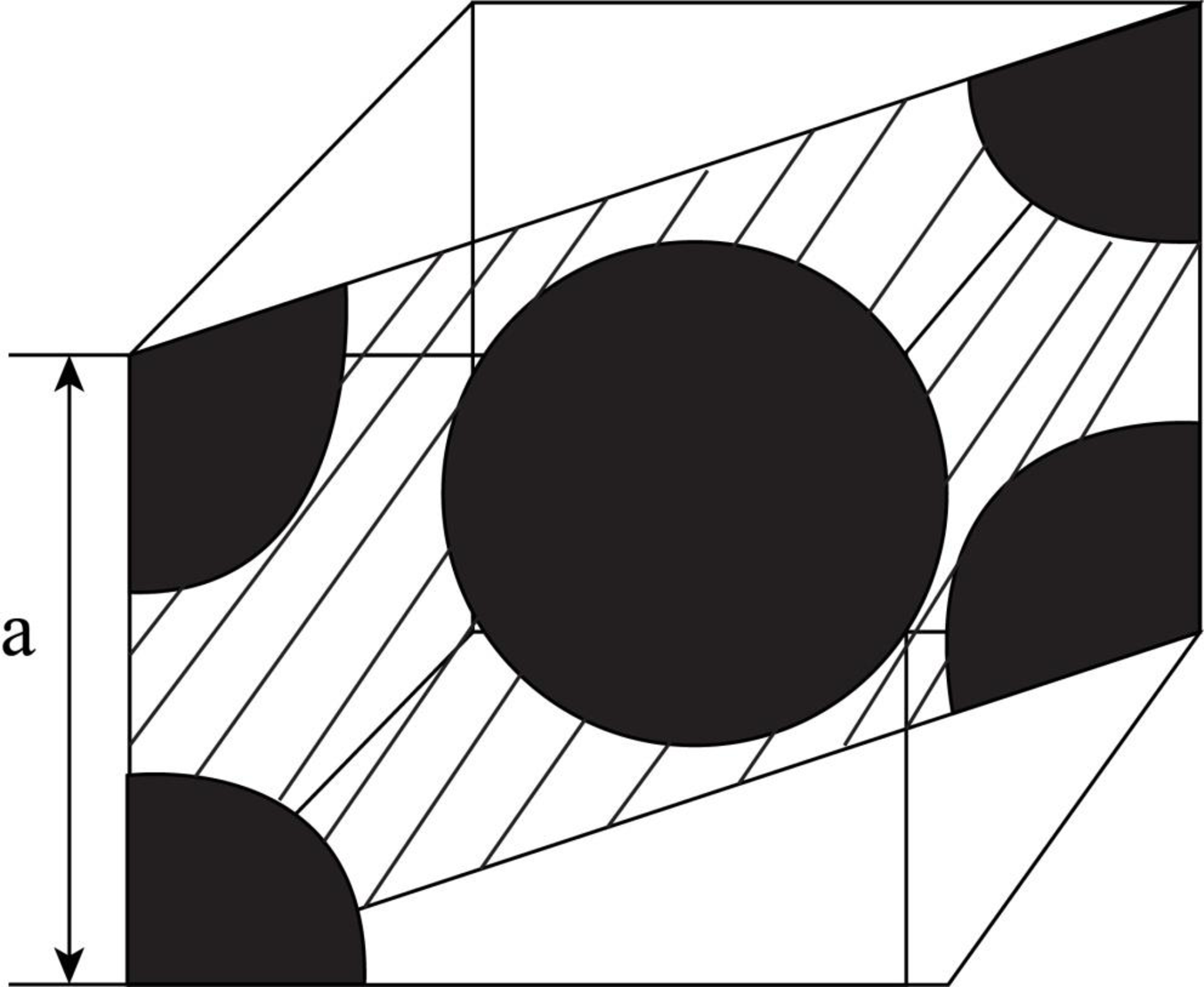
Figure (2)
Write the expression for the planer density of the atom.
Here, the number of the atoms is
Conclusion:
Here, the number of atoms for
Substitute
Thus, the linear atomic density of atom is
(c)
The linear atomic density of atom.
(c)
Answer to Problem 60AAP
The linear atomic density of atom is
Explanation of Solution
Draw the figure for the plane of
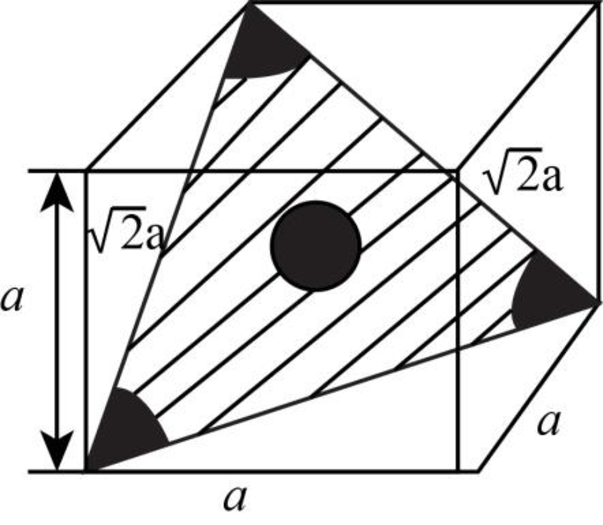
Figure-(3)
Write the expression for the planer density of the atom.
Here, the number of the atoms is
Conclusion:
Here, the number of atoms for
Substitute
Thus, the linear atomic density of atom is
Want to see more full solutions like this?
Chapter 3 Solutions
Foundations of Materials Science and Engineering
- Sketch the lattice planes in cubic crystal using Miller indices for (-200), (-1-20) and (302) planesarrow_forwardIn a cubic crystal, draw any three (3) parallel planes and determine their miller indices.arrow_forwardqjly21 For FCC iron, calculate the diffraction angle for the (220) set of planes. The lattice parameter for Fe is 0.3571 nm. Assume that monochromatic radiation having a wavelength of 0.1540 nm is used, and the order of reflection is 1.arrow_forward
- The density of a sample of HCP beryllium is 1.844 g/cm 3 , and the lattice parameters are a 0 = 0.22858 nm and c 0 = 0.35842 nm. Calculate (a) the fraction of the lattice points that contains vacancies: and (b) the total number of vacancies in a cubic centimetre of Bearrow_forwarda. For a FCC crystal, list all the planes in the {1 2 1} family. b. Would you expect a family in a face-centered orthorhombic (FCO) crystal to have more or fewer planes than the same family in a FCC crystal? Explain your answer.arrow_forwardNickel has the fcc crystal structure and a lattice parameter of a = 0.3517 nm. Using this information, determine the atomic radius of Ni in angstroms.arrow_forward
- With a hexagonal crystal structure where a0=0.22858nm, co=0.35842nm, an atomic radius of 0.1143nm, density of 1.848g/cm3, and an atomic weight of 9.01g/mol, what is a)number of atoms in each unit cell and b) the packing factor in each unit cell.arrow_forwardPlatinum is a metal having a FCC crystal structure with a density p= 21.45 kg/m3. Calculate the lattice constant for this crystal as well as the atomic radius of platinumarrow_forwardExamine your crystal models and find: (a) the number of different (non-parallel) close - packed planes and close - packed directions in the ccp (Cubic closed packing) and hcp (hexagonal tightly packed) structures; and (b) the number of closest - packed planes and close - packed directions in the bcc ( Body - centered cubic) structure.arrow_forward
- (a) For a material having BCC crystal cell structure, write the expressions for Linear atomic density in (1,0,0) and (1,1,0) directions in terms of atomic radius ‘r’ and calculate for Chromium in gms/cm3 . Why metal shows directional nature of properties at atomic level whereas its bulk measured properties are isotropic in nature.arrow_forwardIf the atomic radius of a metal that has the facecentered cubic crystal structure is 0.137 nm,calculate the volume of its unit cell (in nm3).arrow_forwardCalculate the radius of a palladium (Pd) atom, given that Pd has an FCC crystal structure, a density of 12.0 g/cm3 , and an atomic weight of 106.4 g/mol.arrow_forward
 Understanding Motor ControlsMechanical EngineeringISBN:9781337798686Author:Stephen L. HermanPublisher:Delmar Cengage Learning
Understanding Motor ControlsMechanical EngineeringISBN:9781337798686Author:Stephen L. HermanPublisher:Delmar Cengage Learning Welding: Principles and Applications (MindTap Cou...Mechanical EngineeringISBN:9781305494695Author:Larry JeffusPublisher:Cengage Learning
Welding: Principles and Applications (MindTap Cou...Mechanical EngineeringISBN:9781305494695Author:Larry JeffusPublisher:Cengage Learning

