
Concept explainers
(a)
The linear atomic density of atom.
(a)
Answer to Problem 61AAP
The linear atomic density of atom is
Explanation of Solution
Draw the figure for the plane of
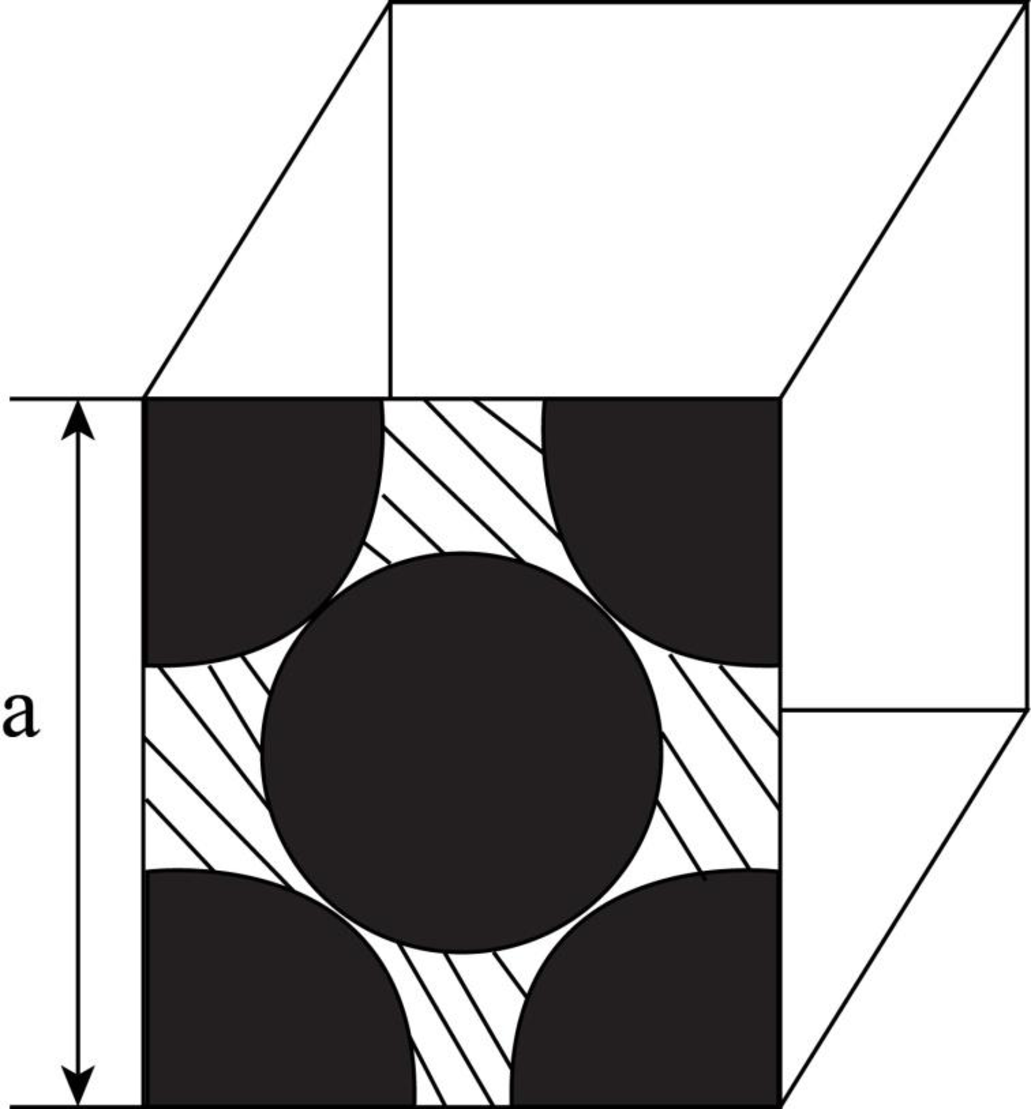
Figure (1)
Write the expression for the planer density of the atom.
Here, the number of the atoms is
Conclusion:
Substitute
Thus, the linear atomic density of atom is
(b)
The linear atomic density of atom.
(b)
Answer to Problem 61AAP
The linear atomic density of atom is
Explanation of Solution
Draw the figure for the plane of
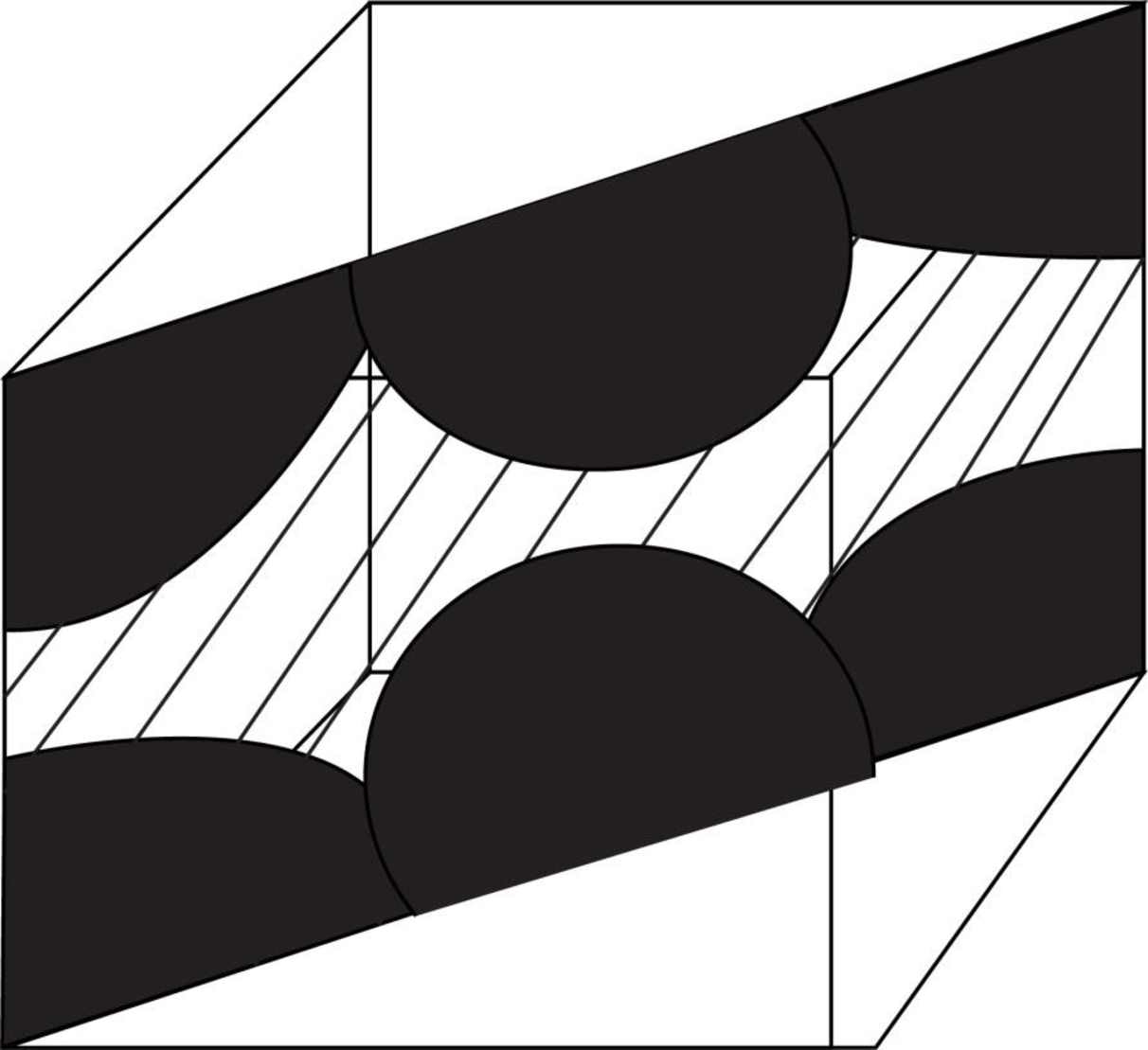
Figure (2)
Write the expression for the planer density of the atom.
Here, the number of the atoms is
Conclusion:
Substitute
Thus, the linear atomic density of atom is
(c)
The linear atomic density of atom.
(c)
Answer to Problem 61AAP
The linear atomic density of atom is
Explanation of Solution
Draw the figure for the plane of
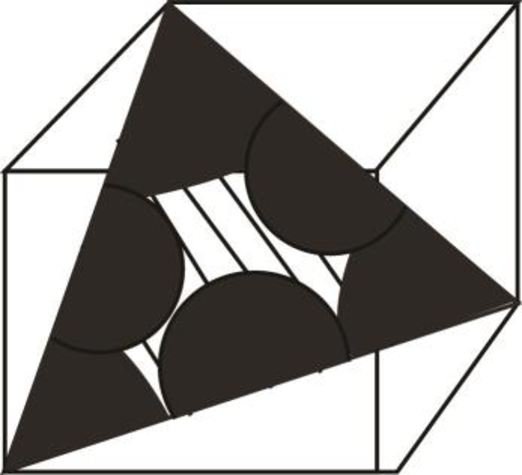
Figure (3)
Write the expression for the planer density of the atom.
Here, the number of the atoms is
Conclusion:
Substitute
Thus, the linear atomic density of atom is
Want to see more full solutions like this?
Chapter 3 Solutions
Foundations of Materials Science and Engineering
- Determine the Miller indices of the cubic crystal plane that intersects the following position coordinates(points): (1, ½, 1), (½, 0, ¾), and (1, 0, ½). Sketch the plane, and show all steps and distances.arrow_forwardGiven that molybdenum (Mo) with atomic weight 95.94 g/mol and density 10.2 g/cm3 assumes a body-centered cubic (BCC) crystal structure, estimate its atomic radius in pm.arrow_forwarda. For a FCC crystal, list all the planes in the {1 2 1} family. b. Would you expect a family in a face-centered orthorhombic (FCO) crystal to have more or fewer planes than the same family in a FCC crystal? Explain your answer.arrow_forward
- The density of a sample of HCP beryllium is 1.844 g/cm 3 , and the lattice parameters are a 0 = 0.22858 nm and c 0 = 0.35842 nm. Calculate (a) the fraction of the lattice points that contains vacancies: and (b) the total number of vacancies in a cubic centimetre of Bearrow_forwardSketch the lattice planes in cubic crystal using Miller indices for (-200), (-1-20) and (302) planesarrow_forwardIn a cubic crystal, draw any three (3) parallel planes and determine their miller indices.arrow_forward
- qjly21 For FCC iron, calculate the diffraction angle for the (220) set of planes. The lattice parameter for Fe is 0.3571 nm. Assume that monochromatic radiation having a wavelength of 0.1540 nm is used, and the order of reflection is 1.arrow_forwardDetermine the possible crystal structure of Zinc Blende structure (ZnS), if the radii are as follows Zn+2 = 0.074 and S-2 = 0.184.arrow_forwardCalculate the number of vacancies per cubic meter for some metal, M, at 811°C. The energy for vacancy formation is 0.89 eV/atom, while the density and atomic weight for this metal are 6.96 g/cm3 (at 811°C) and 55.72 g/mol, respectively.arrow_forward
- Platinum is a metal having a FCC crystal structure with a density p= 21.45 kg/m3. Calculate the lattice constant for this crystal as well as the atomic radius of platinumarrow_forwardCalculate the surface density of the lattice atoms that lie on the (100) plane of an FCC crystal??? Please solve it quickly by handwrittenarrow_forwardCalculate the atomic densities of the directions [100], [111] and [110] in a face centered cubic structure.arrow_forward
 Understanding Motor ControlsMechanical EngineeringISBN:9781337798686Author:Stephen L. HermanPublisher:Delmar Cengage Learning
Understanding Motor ControlsMechanical EngineeringISBN:9781337798686Author:Stephen L. HermanPublisher:Delmar Cengage Learning
