
Concept explainers
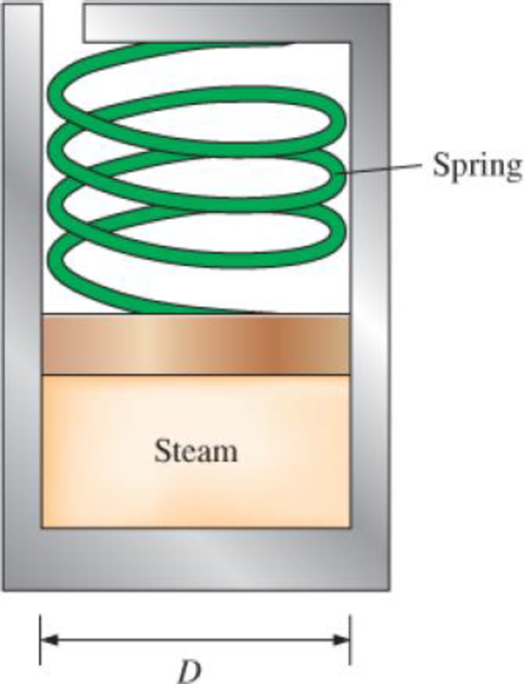
The spring-loaded piston–cylinder device shown in Fig. P3–63 is filled with 0.5 kg of water vapor that is initially at 4 MPa and 400°C. Initially, the spring exerts no force against the piston. The spring constant in the spring force relation F = kx is k = 0.9 kN/cm and the piston diameter is D = 20 cm. The water now undergoes a process until its volume is one-half of the original volume. Calculate the final temperature and the specific enthalpy of the water.
FIGURE P3–63
The final temperature of the spring-loaded piston-cylinder device.
The enthalpy of the spring-loaded piston-cylinder device.
Answer to Problem 63P
The final temperature of the spring-loaded piston-cylinder device is
The enthalpy of the spring-loaded piston-cylinder device is
Explanation of Solution
Write the final temperature of the spring-loaded piston-cylinder device using linear
Here, the spring constant is
Determine the final specific volume of the spring-loaded piston-cylinder device.
Determine the quality of final state for the spring-loaded piston-cylinder device.
Here, the specific volume of saturated liquid is
Determine the enthalpy at the final state of the spring-loaded piston-cylinder device.
Here, the specific enthalpy of saturated liquid is
Conclusion:
From the Table A-6, “Superheated water” to obtain the value of the specific volume of steam at 4 MPa of pressure and
Substitute
Substitute
Refer to Table A-6, “Saturated water”, obtain the below properties at the final pressure
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y are pressure and temperature.
Show the pressure at
| S. No |
Pressure, kPa |
Temperature, |
| 1 | 218.41 | |
| 2 | ||
| 3 | 223.95 |
Calculate final temperature at the pressure
Substitute
From above calculation the final temperature at the pressure
Thus, the final temperature of the spring-loaded piston-cylinder device is
Repeat the above Equation (V), to obtain the value of specific volume of saturated liquid, the specific volume of saturated vapour, specific enthalpy of saturated liquid and the specific enthalpy of saturated vapour at the final pressure
Substitute
Substitute
Thus, the enthalpy of the spring-loaded piston-cylinder device is
Want to see more full solutions like this?
Chapter 3 Solutions
Thermodynamics: An Engineering Approach
- A piston-cylinder device contains 50 kg of water at 250 kPa and 25°C. The cross-sectional area of the plunger is 0.1 m2. Heat is transferred to the water, causing some of it to evaporate and expand; When the volume reaches 0.2 m3, the piston hits a linear spring whose spring constant is 100 kN/m. More heat is transferred to the water until the plunger rises a further 20 cm. Determine a) the final pressure and temperature and b) the work done during this process. Also show the process on a P-V diagram.arrow_forwardA rigid vessel contains contain 5 kg of wet steam at 0.4 MPa. After the addition of 9858 kJ, the steam has a pressure of 0.20 MPa and a temperature of 700C. Determine the initial internal energy and the specific volume of the steam.arrow_forwardA rigid vessel contains 5 kg of wet steam at 0.4 Mpa. After the addition of 9,585 J the steam has a pressure of 2.0 Mpa and a temperature of 700C. Determine the initial internal energy and the specific volume of the steam.arrow_forward
- A piston–cylinder device initially contains 0.6 m3 of saturated water vapor at 250 kPa. At this state, the piston is resting on a set of stops, and the mass of the piston is such that a pressure of 300 kPa is required to move it. Heat is now slowly transferred to the steam until the volume doubles. Show the process on a P-v diagram with respect to saturation lines and determine (a) the final temperature, (b) the work done during this process, and (c) the total heat transfer.arrow_forwardA 12-ft3 rigid tank contains refrigerant-134a at 30 psia and 55 percent quality. Heat is transferred now to the refrigerant from a source at 120°F until the pressure rises to 50 psia. Assuming the surroundings to be at 75°F, determine the amount of heat transfer between the source and the refrigerant.arrow_forwardA rigid vessel of volume 0.02 m3 contains 0.054 kg of steam at an initial pressure of 4 bar. Heat is supplied to the steam from an external source. Calculate (i) the initial quality of the steam, (ii) the temperature and quality of the steam when the pressure becomes 4.5 bar, (iii) the pressure of the steam when it is just dry saturated, and (iv) the temperature when the pressure reaches 7 bar. Indicate the process on a P-V and T-V diagram for water. [Answers: (i) 0.8, (ii) 147.9°C, 0.894, (iii) 5.07 bar, (iv) 298.4°C]arrow_forward
- A mass of 10 g of oxygen fill a weighted piston– cylinder device at 20 kPa and 100°C. The device is now cooled until the temperature is 0°C. Determine the change of the volume of the device during this coolingarrow_forwardThe piston-cylinder system shown above contains R-410a at 20°C and 100 kPa. If the temperature of the R-410a is increased to 50°C the specific volume in the piston-cylinder is ?arrow_forwardDetermine the quality of steam at 169.06 kPa when 270 kJ/kg of energy are lost from saturated steam. What is the steam temperature?arrow_forward
- Q9/ A piston-cylinder device contains 0.85 kg of refrigerant- 134a at 10°C. The piston that is free to move has a mass of 12 kg and a diameter of 25 cm. The local atmospheric pressure is 88 kPa. Now, heat is transferred to refrigerant-134a until the temperature is 15°C. Determine (a) the final pressure, (b) the change in the volume of the cylinder, and (e) the change in the enthalpy of the refrigerant-134a.arrow_forwardA frictionless piston–cylinder device contains 10 lbm of steam at 60 psia and 320°F. Heat is now transferred to the steam until the temperature reaches 400°F. If the piston is not attached to a shaft and its mass is constant, determine the work done by the steam during this process.arrow_forward0.03-m of air is contained in the spring-loaded piston-cylinder device shown in Fig. P4-53. The spring constant is 875 N/m, and the piston diameter is 25 cm. When no force is exerted by the spring on the piston, the state of the air is 2250 kPa and 240°C. This device is now cooled until the vol- ume is one-half its original size. Determine the change in the internal energy and enthalpy.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





