
Concept explainers
The police often use a device called a Breatkalyzer to test drivers suspected of being drunk. In one type of device, the breath of a driver suspected of driving under the influence of alcohol is bubbled through an orange solution containing potassium dichromate
(a) Classify each of the species in the Breathalyzer reaction as a strong electrolyte, weak electrolyte, or nonelectrolyte, (b) Write the ionic and net ionic equations for the Breathahzer reaction. (c) Determine the oxidation number of each element in the overall equation. (d) One manufacturer of Breathalyzers specifies a potassium dichromate concentration of 0.025 percent weight per volume
Interpretation:
The reactants and products in breathalyzer are to be classified as strong, weak, or non-electrolyte, the ionic and the net ionic equation for the reaction are to be represented, and the oxidation state of each element is to be determined in the reaction. The concentration of potassium dichromate in molarity is to be expressed and the volume of stock solution of
Concept introduction:
An electrolyte is a compound which dissociates into its corresponding ions when dissolved in water and conducts electricity. Electrolytes can be strong, weak, or non-electrolyte. The classification of the type of electrolyte is based on the formation of the ions when the electrolyte is dissolved in water.
An ionic reaction always follows the law of conservation of mass, according to which, when a chemical reaction occurs, the mass of ions in products should be equal to the mass of ions in reactants.
Oxidation number is the net charge on an element involved in the formation of a compound in a reaction. It is also known as oxidation state.
The concentration of a solution in terms of molarity is determined as follows:
Here,
Dilution is the process by which a less concentrated solution can be prepared from a more concentrated solution. But, the number of moles of solute remains the same in the original solution and the dilution. So, the concentration or volume of dilution can be determined as follows:
Here,
in ml,
Answer to Problem 159AP
Solution:
a)
b)
Ionic equation for breathalyzer is as follows:
The net ionic equation is as follows:
c)
Oxidation states of reactants and products are:
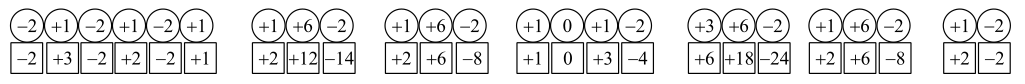
d)
The molar concentration of
e)
The volume of
f)
The molarity of
Explanation of Solution
a)
Given information: The reaction for breathalyzer is as follows:
a) Classify each of the species in the Breathalyzer reaction as a strong electrolyte, weak electrolite or nonelectrolyte.
b) The ionic and net ionic equations for the Breathalyzer reaction.
The ionic equation for the breathalyzer reaction is as follows:
The net ionic equation for the breathalyzer reaction is as follows:
c) The oxidation number of each element in the overall equation.
The oxidation numbers of elements in
The oxidation numbers of elements in
The oxidation numbers of elements in
The oxidation numbers of elements in
The oxidation numbers of elements in
The oxidation numbers of elements in
The oxidation numbers of elements in
d) The concentration in terms of molarity for the reaction
The concentration of
The molecular weight of
Substitute the values in the equation as follows:
e) The volume of
The concentration of stock solution is
Consider the two solutions, the stock solution is solution
Rearranging the equation to calculate the volume as follows:
f) The molarity of each ion in a
Potassium dichromate form the following ions on dissolution:
So, the concentration of these ions is as follows:
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry
Additional Science Textbook Solutions
Introduction to Chemistry
Chemistry: A Molecular Approach
Inorganic Chemistry
Chemistry: The Molecular Nature of Matter
- A 1.345-g sample of a compound of barium and oxygen was dissolved in hydrochloric acid to give a solution of barium ion, which was then precipitated with an excess of potassium chromate to give 2.012 g of barium chromate, BaCrO4. What is the formula of the compound?arrow_forward3.14 A number of compounds are used in cement, and reactions among them occur when water is added. In one, CaO reacts with Al2O3 and water to form Ca3Al2(OH)12. Write a bal- anced chemical equation for this process.arrow_forwardDetermine the volume of sulfuric acid solution needed to prepare 37.4 g of aluminum sulfate, Al2(SO4)3, by the reaction 2Al(s)+3H2SO4(aq)Al2(SO4)3(aq)+3H2(g) The sulfuric acid solution, whose density is 1.104 g/mL, contains 15.0% H2SO4 by mass.arrow_forward
- Vitamin C is ascorbic acid, HC6H7O6, which can be titrated with a strong base. HC6H7O6(aq) + NaOH(aq) NaC6H7O6(aq) + H2O() A student dissolved a 500.0-mg vitamin C tablet in 200.0 mL water and then titrated it with 0.1250-M NaOH. It required 21.30 mL of the base to reach the equivalence point. Calculate the mass percentage of the tablet that is impurity.arrow_forwardYou can dissolve an aluminum soft drink can in an aqueous base such as potassium hydroxide. 2 Al(s) + 2 KOH(aq) + 6 H2O(l) 2 KAI(OH)4(aq) + 3 H2(g) If you place 2.05 g of aluminum in a beaker with 185 mL of 1.35 M KOH, will any aluminum remain? What mass of KAI(OH)4 is produced?arrow_forwardA 300.0-g sample of a solid is made up of a uniform mixture of NaNO3, MgCl2, and BaCl2. A 100.0-g sample of the mixture is dissolved in water and treated with an excess of KOH. The precipitate from the reaction has a mass of 13.47 g. The remaining 200.0-g sample is also dissolved in water and treated with an aqueous solution of AgNO3. The resulting precipitate has a mass of 195.8 g. What are the masses of NaNO3, MgCl2, and BaCl2 in the 300.0-g sample?arrow_forward
- Magnesium metal (a component of alloys used in aircraft and a reducing agent used in the production of uranium, titanium, and other active metals) is isolated from sea water by the following sequence of reactions: Mg2+(aq)+Ca(OH)2(aq)Mg(OH)2(s)+Ca2+(aq)Mg(OH)2(s)+2HCl(aq)MgCl2(s)+2H2O(l)MgCl2(l)electrolysisMg(s)+Cl2+Cl2(g) Sea water has a density of 1.026 g/cm3 and contains 1272 parts per million of magnesium a5 Mg2+(aq) by mass. What mass, in kilograms, of Ca(OH)2; is required to precipitate 99.9% of the magnesium in 1.00103 L of sea water?arrow_forwardTwenty-five mL of a 0.388 M solution of Na2SO4 is mixed with 35.3 mL of 0.229 M Na2SO4. What is the molarity of the resulting solution? Assume that the volumes are additive.arrow_forwardLactic acid, C3H6O3 is the acid present in sour milk. A 0.100-g sample of pure lactic acid requires 12.95 mL of 0.0857 M sodium hydroxide for complete reaction. How many moles of hydroxide ion are required to neutralize one mole of lactic acid?arrow_forward
- When 10. L of water is added to 3.0 L of 6.0 M H2SO4, what is the molarity of the resulting solution? Assume the volumes are additive.arrow_forwardThioridazine, C21H26N2S2, is a pharmaceutical agent used to regulate dopamine (Dopamine, a neurotransmitter, affects brain processes that control movement emotional response, and ability to experience pleasure and pain.) A chemist can analyze a sample of the pharmaceutical for the thioridazine content by decomposing it to convert the sulfur in the compound to sulfate ion. This is then trapped as water-insoluble barium sulfate (see Figure 4.4). SO42(aq, from thioridazine) + BaCl2(aq) BaSO4(s) + 2 Cl(aq) Suppose a 12-tablet sample of the drug yielded 0.301 g of BaSO4. What is the thioridazine content, in milligrams, of each tablet?arrow_forwardOn Easter Sunday, April 3, 1983, nitric acid spilled from a tank car near downtown Denver, Colorado. The spill was neutralized with sodium carbonate: 2HNO3(aq)+Na2CO3(aq)2NaNO3(aq)+H2O(l)+CO2(g) a. Calculate H for this reaction. Approximately 2.0 104 gal nitric acid was spilled. Assume that the acid was an aqueous solution containing 70.0% HNO3 by mass with a density of 1.42 glcm3. What mass of sodium carbonate was required for complete neutralization of the spill, and what quantity of heat was evolved? (Hf for NaNO3(aq) = 467 kJ/mol) b. According to The Denver Post for April 4, 1983, authorities feared that dangerous air pollution might occur during the neutralization. Considering the magnitude of H, what was their major concern?arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





