
Biochemistry
6th Edition
ISBN: 9781305577206
Author: Reginald H. Garrett, Charles M. Grisham
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 15P
Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book.
Understanding the Chemistry of the Dipeptide Sweetener Aspartame
The artificial sweeteners Equal and Nutrasweet contain aspartame, which has the structure:
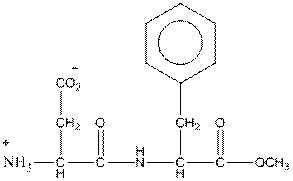
What are the two amino acids a that are components of aspartame? What kind of bond links these amino acids? What do you suppose might happen if a solution of aspartame was healed for several hours at a pH near neutrality? Suppose you wanted to make hot chocolate sweetened only with aspartame, and you stored it in a thermos for several hours before drinking it. What might it taste like?
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 4 Solutions
Biochemistry
Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...
Ch. 4 - Prob. 11PCh. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...Ch. 4 - Answers to all problems are at the end of this...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Answers to all problems are at the end of this book Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Solving the Sequence of an Oligopeptide From Sequence Analysis Data Analysis of the blood of a catatonic football fan revealed large concentrations of a. psychologic octapeptide. Amino acid analysis of this oclapeplide gave the following results: 2 Ala lArg 1 Asp 1 Mel 2 Tyr I Val 1NH/ The following facts were observed: Partial acid hydrolysis of the octapeptide yielded a dipeptide of the structure Chymolrypsin treatment of the octapeplide yielded two tetrapeptides, each containing an alanine residue. Trypsin treatment of one of the tetrapeptides yielded two dipeptides. Cyanogen bromide treatment of another sample of the same tetrapeplide yielded a tripeplideand free Tyr. N-lerminal analysis of the other tetrapeptide gave Asn. What is the amino acid sequence of this oclapeplide?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Writing Dissociation Equations for Amino Acids Write equations fur the ionic dissociations of alanine, glutamate, histidine, lysine, and phenylalanine.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Solving the Sequence of an Oligopeptide From Sequence Analysis Data Amino acid analysis of a decapeptide revealed the presence of the following products: The following facts were observed: Neither car boxy peptidase A nor B treatment of the- decapeptide had any effect. Trypsin treatment yielded two tetrapcptides and free Lys. Clostripain treatment yielded a tetrapcptide and a hexapeptidc. Cyanogen bromide treatment yielded an octapeptide and a dipeptide of sequence NP (using the one-letter codes). Chymotrypsin treatment yielded two tripeptides and a telrapeptide. The N-terminal chymotryptic peptide had a net charge of — 1 at neutral pi I and a net charge of —3 al pH 12. One cycle of Ed man degradation gave the PTH derivative What is the ammo acid sequence of this decapeptide?arrow_forward
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Solving the Sequence of an Oligopeptide From Sequence Analysis Data Amino acid analysis of ail oligopeptide seven residues long gave The following fads were observed: a. Trypsin treatment had no apparent effect. b. The phenylthiohydantoin released by Lid mini degradation was c. Brief chymotrypsin treatment yielded several products, including a dipeptide and a tetrapeptide. The amino acid composition of the tetrapeptide was Leu, Lyi. and Met. d. Cyanogen bromide treatment yielded a dipeptide, a tetrapeptide, and free Lys. What is the amino acid sequence of this heptapeptide?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Draw the Titration Curve for a Weak Acid and Determine its pKa from the Titration Curve When a 0.1 M solution of a weak acid was titrated with base, the following results were obtained: Plot the results of this titration and determine the pK a of the weak acid from your graph.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Calculating pH in Amino Acid Solutions II (Integrates with Chapter 2.) Calculate the pH at which the -amino group of lysine is 20% dissociated.arrow_forward
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. The Role of Proline Residues in -Turns Pro is the amino acid least commonly found in «-helices but most commonly found in -turns. Discuss the reasons for this behavior.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Assessing the pH Dependence of Poly-L-Glutamate Structure Poly-L glutamate adopts an tr-helical structure at low pH but becomes a random coil above pH 5. Explain this behavior.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Use examples from the ActiveModel for Human GaleLtin-1 to describe the hydrophobic effect.arrow_forward
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Proteins and nucleic acids are informational macromolecules. What are the two minimal criteria for a linear informational polymer?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. A Rule of Thumb for Amino Acid Content in Proteins The simple average molecular weight of the 20 common amino adds is 138, but most biochemists use 110 when estimating the number of amino acids in a protein of known molecular weight. Why do you Suppose this is? (Hint: There are two contributing factors to the answer. One of them will be apparent from a brief consideration of the amino acid compositions of common proteins. See, for example, Figure 5.16 of this text.)arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. The Structure and Ionization Properties of Nucleotides Draw the principal ionic species of occurring at pH 2.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning
Chapter 7 - Human Movement Science; Author: Dr. Jeff Williams;https://www.youtube.com/watch?v=LlqElkn4PA4;License: Standard youtube license