
Concept explainers
Interpretation:
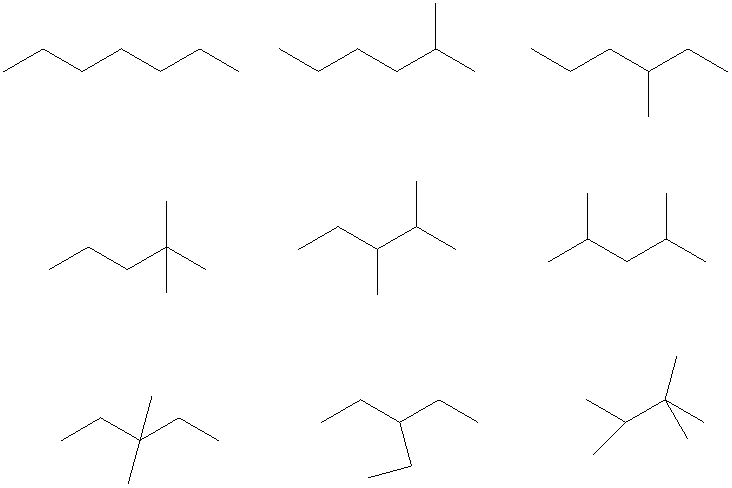
The condensed and bond-line structural formula for all the constitutional isomers with molecular formula
Concept introduction:
Constitutional isomers are the isomers in which the molecular formula remains same but the arrangements of groups or atoms in the structure are different.
Mostly organic compound is represented by the bond line structural formulas.
In bond line formula each bond is represented by a line.
At the end of each line if no atom is shown than a carbon atom is present.
Condensed structural formula is defined as the formula in which all the carbon-carbon bonds are eliminated and the hydrogen atom written just after the carbon atom.
Answer to Problem 1PP
Solution:
Bond-line structural formula:
Condensed formula:
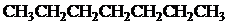
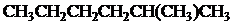
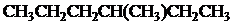
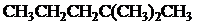
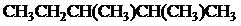
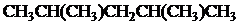
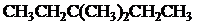
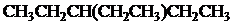
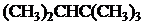
Explanation of Solution
Given: The molecular formula is
In

All the above structures have same molecular formula,
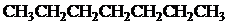
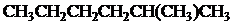
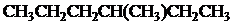
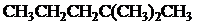
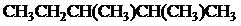
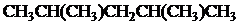
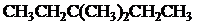
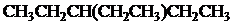
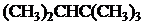
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry
- Identify the structures of isomers A and B (molecular formula C9H10O).arrow_forwardDraw line-angle formulas for the three constitutional isomers with the molecular formula C5H12.arrow_forwarda. For each disubstituted cyclohexane below, draw its ring-flip isomer. Circle the most stable conformation and label the substituent groups as axial or equatorial. ( see image) b.Draw and name the seven constitutional isomers (all contain a ring of some size) for cycloalkane, C6H12.arrow_forward
- 1. Write bond-line formulas for (a.) four aldehydes with the formula of C5H10O (b) three ketones that have the formula C5H10O (c.) four carboxylic acids with the formula C5H10O2 (d.) three esters with the formula C5H10O2arrow_forwardAcetylcholine is a neurotransmitter in the central nervous system in humans. Sighting along the C-C bond, draw Newman projection formulas for all the eclipsed and staggered conformers of acetylcholine. Which among the conformers is the least stable? The most stable? Plot a conformational analysis for these conformers.arrow_forwardDraw 3-D structures for all possible constitutional isomer and Write IUPAC names and include cis-trans a) )(Only three-membered rings) C3H6, C3H5CL, and C3H4CL2 b)(Only five-membered rings) C5H10, C5H9CL and C5H8CL2arrow_forward
- For 1,2-dichloroethane: ( Q.) Draw Newman projections for all eclipsed conformations formed by rotation from 0° to 360° about the carbon-carbon single bond.arrow_forward5. Draw all the structures of constitutional isomers with the molecular formula:a) C4H6b) C4H9Brc) C4H10Oarrow_forwardHow would you best describe the C-C bonds lengths in benzene relative to cyclohexane? Hypothesize why these results are observed.arrow_forward
- Is the geometry of CH2BrCl, the same as CH3Cl and CH4? Briefly explain the evidence for your answerarrow_forwardtranslate the bond-line notation structure to the Newman projection by filling int the missing groups (A, B, C, D or E) on the lines in the Newman projection so they match the conformation given in the original structure. Circle if the conformation is a staggered or eclipsed.arrow_forwardDraw all the isomers with molecular formula C6H12 that contain a cyclobutane ring. (Hint: There are seven.)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





