
Concept explainers
Interpretation:
The chirality of isomeric alcohol having molecular formula
Concept introduction:
A chiral carbon atom is one which is attached to four different atoms or groups.
Different compounds having the same molecular formula are called isomers. They may differ in their constitution or arrangement of atoms in space.
Answer to Problem 26P
Solution:
Isomeric alcohols having the molecular formula

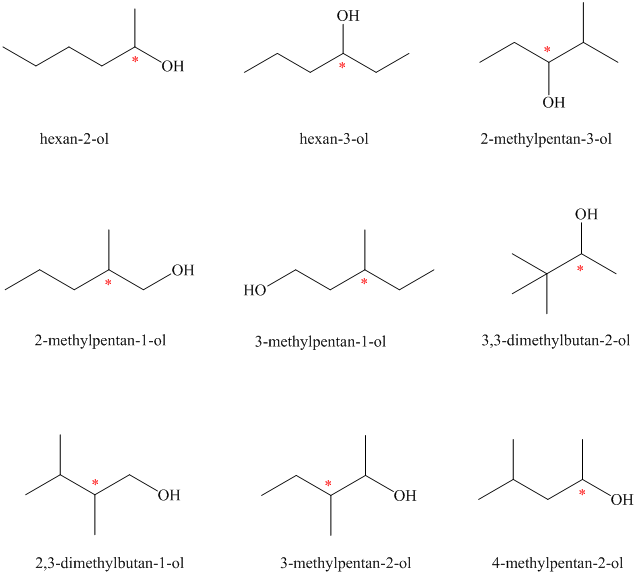
Isomeric alcohols having the molecular formula
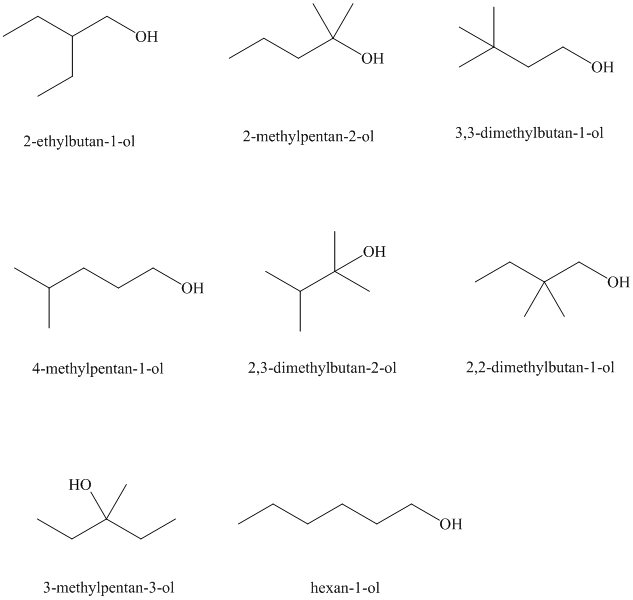
Explanation of Solution
There are
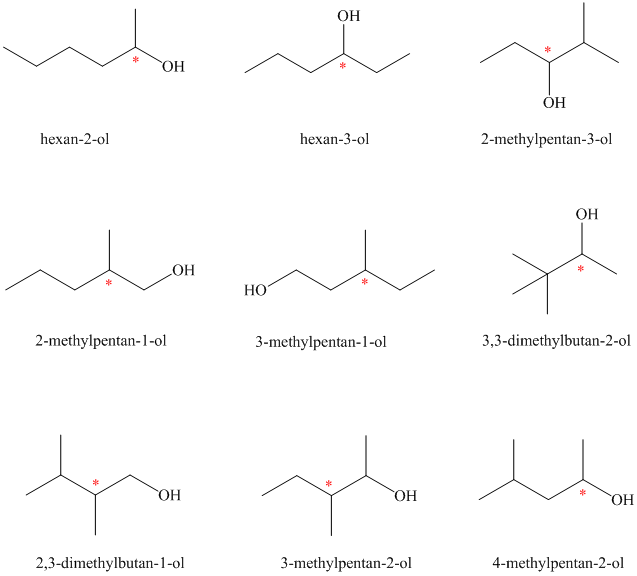
The remaining
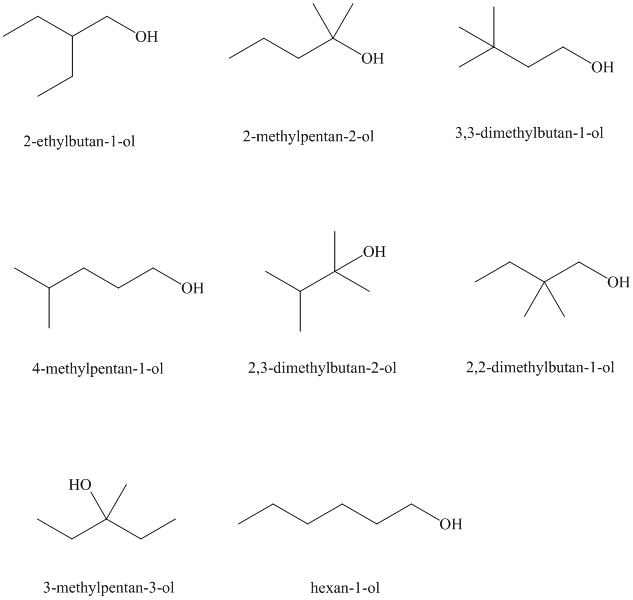
An alcohol is said to be chiral if a carbon atom in it is attached to four different atoms or groups.
Want to see more full solutions like this?
Chapter 4 Solutions
Loose Leaf for Organic Chemistry
- Draw the products formed when (S)-butan-2-ol is treated with TsCl and pyridine, followed by NaOH. Label the stereogenic center in each compound as R or S. What is the stereochemical relationship between the starting alcohol and the nal product?arrow_forwardFive isomeric alkanes (A–E) having the molecular formula C6H14 are each treated with Cl2 + hν to give alkyl halides having molecular formula C6H13Cl. A yields five constitutional isomers. B yields four constitutional isomers. C yields two constitutional isomers. D yields three constitutional isomers, two of which possess stereogenic centers. E yields three constitutional isomers, only one of which possesses a stereogenic center. Identify the structures of A–E.arrow_forwardThe reaction of 4-methylcyclohexanone with CH3MgBr followed by neutralization gives two alcohols. These two alcohols are A. enantiomers formed in equal amounts. B. diastereomers. C. constitutional isomers. D. enantiomers formed in unequal amounts.arrow_forward
- Five isomeric alkanes (A–E) having the molecular formula C6H14 are each treated with Cl2 + hv to give alkyl halides having molecular formulaC6H13Cl. A yields five constitutional isomers. B yields four constitutionalisomers. C yields two constitutional isomers. D yields threeconstitutional isomers, two of which possess stereogenic centers. Eyields three constitutional isomers, only one of which possesses astereogenic center. Identify the structures of A–E.arrow_forwardAccount for the regioselectivity and stereoselectivity observed when 1-methylcyclopentene is treated with reagent. Q) Br2 in H2Oarrow_forwardTwo diastereomeric sets of enantiomers, A/B and C/D, exist for 3-bromo-2-butanol. When enantiomer A or B is treated with HBr, only racemic 2,3-dibromobutane is formed; no meso isomer is formed. When enantiomer C or D is treated with HBr, only meso 2,3-dibromobutane is formed; no racemic 2,3-dibromobutane is formed. Account for these observations.arrow_forward
- Draw and name the seven aldehydes and ketones with the formula C5H10O. Which are chiral?arrow_forward(i) What is meant by chirality of a compound? Give an example.(ii) Which one of the following compounds is more easily hydrolyzed by KOH and why?CH3CHClCH2CH3 or CH3CH2CH2Clarrow_forwardAre the following molecules chiral? Draw the skeletal structures of each and label the chirality center(s) if present. 1) 2-chloro-4-ethylhepatane 2) cis-1,2-pentadiool (suffix -ol indicates an alcohol) 3) 3,3-dibromo-5-propyloctanearrow_forward
- Thuggacin C is a natural product with excellent biological activity. How many chirality centers does Thuggacin C have? a. 6 b. 7 c. 8 d. 9arrow_forwardTreatment of compound A (C8H17Br) with NaOCH2CH3 affords two constitutional isomers B and C. Ozonolysis of B affords CH2=O and (CH3CH2CH2)2C=O. Ozonolysis of C affords CH3CH2CH2COCH3 and CH3CH2CHO. What is the structure of A?arrow_forwardDraw the structural formula for at least one bromoalkene with the molecular formula C5H9Br that shows: Q.Chirality but not E,Z isomerismarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

