
(a)
Interpretation:
The formula for barium chloride should be written along with identify whether it is soluble in water or not.
Concept introduction:
First, the symbol of the metal (cation) with its ion charge as a superscript should be written.
The symbol of the non-metal (anion) with its ion charge or polyatomic ion as a superscript should be written.
After that, the charges should be criss cross so that they become subscript for the opposite element and + and - charges should be removed.
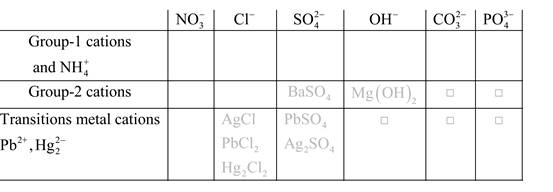
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
(b)
Interpretation:
The formula for magnesium hydroxide should be written along with identify whether it is soluble in water or not.
Concept introduction:
First, the symbol of the metal (cation) with its ion charge as a superscript should be written.
The symbol of the non-metal (anion) with its ion charge or polyatomic ion as a superscript should be written.
After that, the charges should be criss cross so that they become subscript for the opposite element and + and - charges should be removed.
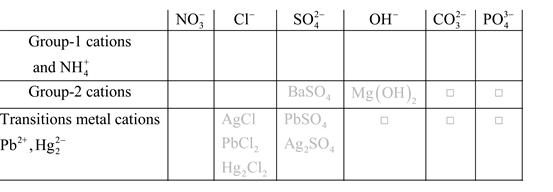
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
(c)
Interpretation:
The formula for chromium(III) carbonate should be written along with identify whether it is soluble in water or not.
Concept introduction:
First, the symbol of the metal (cation) with its ion charge as a superscript should be written.
The symbol of the non-metal (anion) with its ion charge or polyatomic ion as a superscript should be written.
After that, the charges should be criss cross so that they become subscript for the opposite element and + and - charges should be removed.
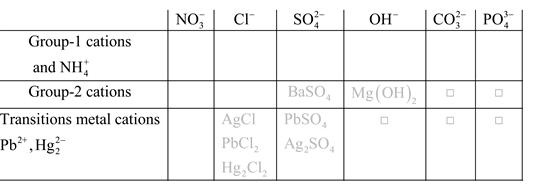
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
(d)
Interpretation:
The formula for potassium phosphate should be written along with identify whether it is soluble in water or not.
Concept introduction:
First, the symbol of the metal (cation) with its ion charge as a superscript should be written.
The symbol of the non-metal (anion) with its ion charge or polyatomic ion as a superscript should be written.
After that, the charges should be criss cross so that they become subscript for the opposite element and + and - charges should be removed.
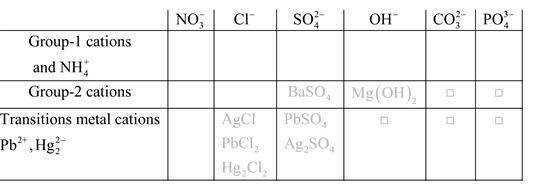
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Chemistry: Principles and Reactions
- Explain why some electrolyte solutions are strongly conducting, whereas others are weakly conducting.arrow_forwardA 1.345-g sample of a compound of barium and oxygen was dissolved in hydrochloric acid to give a solution of barium ion, which was then precipitated with an excess of potassium chromate to give 2.012 g of barium chromate, BaCrO4. What is the formula of the compound?arrow_forwardSodium hydroxide is added to phosphoric acid.arrow_forward
- 4.22 Generally, an excess of O2 is needed for the reaction Sn+O2SnO2 . What is the minimum number of moles of oxygen required to oxidize 7.3 moles of tin?arrow_forwardA 2.5624-g sample of a pure solid alkali metal chloride is dissolved in water and treated with excess silver nitrate. The resulting precipitate, filtered and dried, weighs 3.03707 g. What was the percent by mass of chloride ion in the original compound? What is the identity of the salt?arrow_forwardMagnesium metal (a component of alloys used in aircraft and a reducing agent used in the production of uranium, titanium, and other active metals) is isolated from sea water by the following sequence of reactions: Mg2+(aq)+Ca(OH)2(aq)Mg(OH)2(s)+Ca2+(aq)Mg(OH)2(s)+2HCl(aq)MgCl2(s)+2H2O(l)MgCl2(l)electrolysisMg(s)+Cl2+Cl2(g) Sea water has a density of 1.026 g/cm3 and contains 1272 parts per million of magnesium a5 Mg2+(aq) by mass. What mass, in kilograms, of Ca(OH)2; is required to precipitate 99.9% of the magnesium in 1.00103 L of sea water?arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





