
Concept explainers
Interpretation:
The relationship between the molecules in each pair is to be determined. Whether drawings represent constitutional isomers, stereoisomers, or just different ways of drawing the same compound is to be determined. If they are stereoisomers, it is to be determined if they are enantiomers or diastereomers.
Concept introduction:
Constitutional isomers are compounds with the same chemical formula but different connectivity.
Stereoisomers are compounds with the same chemical formula and connectivity but different spatial arrangement.
If a pair of stereoisomers forms an object and non-superimposable mirror image, then they are called enantiomers. Enantiomers contain a chiral center.
If a pair of stereoisomers does not have an object and its mirror image relation, then they are called diastereomers.
Identical molecules have the same absolute configuration at each chiral center.
Answer to Problem 38P
Solution:
a) These two structures are enantiomers.
b) These are different ways of drawing the same compound.
c) These structures are enantiomers.
d) These structures are constitutional isomers.
e) These are different ways of drawing the same compound.
f) These are different ways of drawing the same compound.
g) These structures are enantiomers.
h) These are diastereomers.
i) These structures are different ways of drawing the same compound.
j) These structures are different ways of drawing the same compound.
Explanation of Solution
a) The two given structures are as follows:
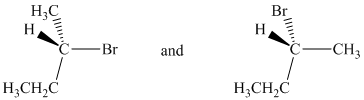
Both structures are chiral as four different atoms/groups attached to the central carbon. The absolute configurations at the chiral center for the two structures are determined from Cahn-Ingold-Prelog (CIP) system.
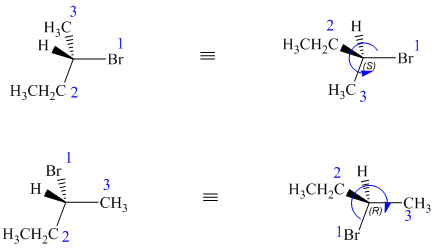
In both structures, the lowest priority group (H) is at the front. The structures are first rotated so that the hydrogen is at the back. The order of priority in the molecule is
In the first structure, the three highest ranking groups trace a counterclockwise path, giving an absolute configuration S.
In the second structure, the three highest ranking groups trace a clockwise path, giving an absolute configuration R.
Since the two structures have different absolute configurations, they are enantiomers.
b) The structures have a plane of symmetry, passing through the atoms (marked in blue) on the horizontal bonds. Rotating any one structure through

c) The two given structures are as follows:
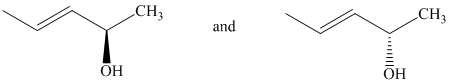
Both the given structures contain one chiral center with identical substituents.
Based on CIP system, the order of priority of the groups attached to the chiral center is

In the first structure, the hydrogen is at the back. The three highest ranking groups trace a clockwise path, giving an absolute configuration of R.
The second structure first needs to be rotated around the horizontal axis so that the hydrogen is at the back. With this, the other three groups follow a counterclockwise path, giving an absolute configuration of S.

Since the two structures have different absolute configurations at the identical chirality centers, these are enantiomers of each other.
d) The two given structures are as follows:
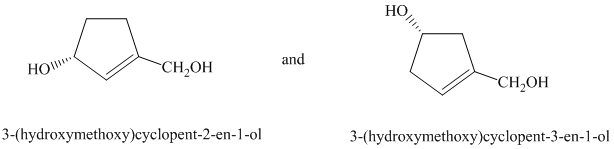
In the first structure, the carbon atom to which the hydroxyl group is attached is numbered first
In the second structure, the carbon atom to which the hydroxyl group is attached is numbered first
In both the given structures, the position of the double bond is different. Thus, the given drawings represent two different compounds with the same molecular formula but with different connectivity of atoms. Thus, they are constitutional isomers of each other.
e) These structures are identical and show different ways of drawing the same compound.

The structures have a plane of symmetry, passing through
f) The given drawings are as follows:
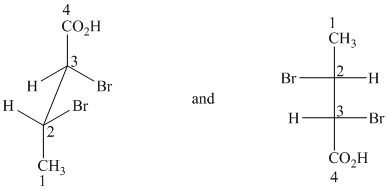
These are different ways of drawing the same compound as shown below:
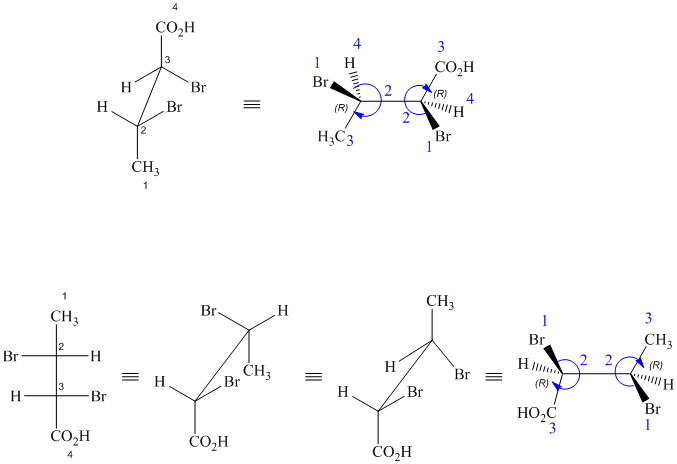
Both structures contain two chiral centers, at
Before comparing, the two structures are redrawn using wedge-dash bonds.
Following the CIP system shows that the two chiral centers in both structures have the same absolute configurations (2R, 3R). Therefore, they are identical and indicate different ways of drawing the same compound.
g) These structures are enantiomers.
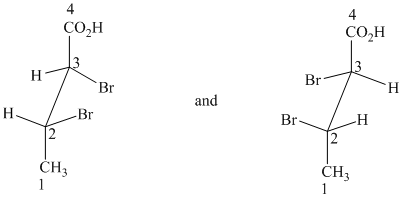
The two drawings contain two chirality centers each. Absolute configurations at each chirality centers for these are shown below:
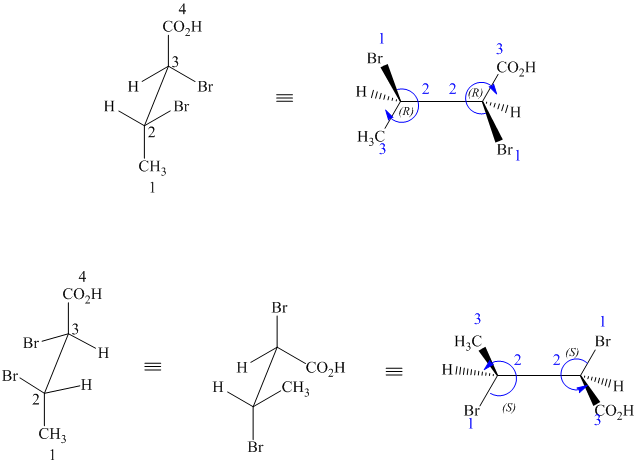
Both structures have two chiral centers,
Based on the CIP system, the absolute configurations of the two centers in the first structure are (2R, 3R).
The absolute configurations of the two centers in second structure are (2S, 3S).
As the absolute configurations at both the centers are changed, the structures are mirror images and therefore enantiomers.
h) These structures are diastereomers – cis-trans isomers.
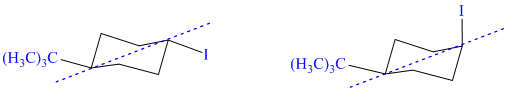
Both structures have two chiral centers. However, they also have a plane of symmetry, one that contains the isopropyl group, iodine atom, and the ring carbons to which these are bonded. Because of this plane of symmetry, these are not chiral molecules; they are diastereomers.
Additionally the iodine atom has different orientations in the two structures with respect to the isopropyl substituent. In the first structure, it is in the equatorial position, and in the second structure it is in the axial position. The isopropyl group is in the equatorial position in both. As a result, the isopropyl group and iodine are trans to each other in the first structure. They are cis to each other in the second structure.
Therefore, these two structures are diastereomers and show cis-trans isomerism.
i) The given pair of compounds are as follows:

In the first structure, the double bonded carbon atoms are
j) These structures are different ways of drawing the same compound.
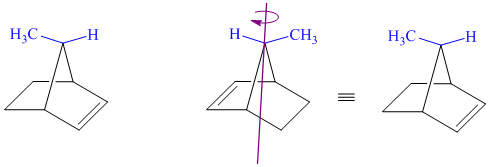
Rotating one of the structures through
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry - Standalone book
- What is the relationship of the following molecule? Are they identical, constituional isomers, enantiomers, or diastereomers?arrow_forwardLabel the following as enantiomers, diastereomers, constitutional isomers, or the same compound.arrow_forwardIdentify pairs of molecules that represent enantiomers and diastereomers and identify each center stereogenic by writing R or S next to it.arrow_forward
- Are the compounds presented conformational isomers, meso compounds, enantiomers, diastereomers, or constitutional isomers? Explain.arrow_forwardDraw all the possible stereoisomers for each compound, and label pairs of enantiomers and diastereomers.arrow_forwardFor each of the following pairs, please indicate if the compounds are (A) identical, (B) constitutional isomers, (C) enantiomers, or (D) diastereomers:arrow_forward
- Explain that how these pairs are identical, enantiomers, diastereomers, or constitutional isomers?arrow_forwardDetermine each as enantiomers, diastereomers, constitutional isomers, or the same molecule.arrow_forwardPart D. Do the two structures A and B of each pair drawn below represent the same molecule, constitutional isomers, or stereoisomers? If A and B are stereoisomers, further classify them as enantiomers or diastereomers.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
