
Concept explainers
What is the geometry around the central atom in the following molecular models? (There are no “hidden” atoms: all atoms in each model are visible.)
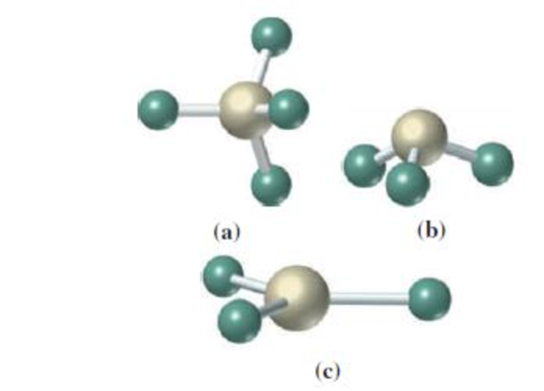
(a)
Interpretation:
The geometry around the central atom in the given molecular model is to be determined.
Concept introduction:
Molecular shape can be predicted from the Lewis structure by using the valence-shell Electron-pair repulsion (VSEPR) model.
- Count the number of valence electron pairs (bond pairs and lone pairs).
- Assume that the valence electron pairs form a structure that allows them to be as far away from each other as possible.
- If there are only two bond pair electrons, the molecule is linear.
- If there are three bond pair electrons, the molecule is shaped like a trigonal planar.
- If there are four bond pair electrons, the molecule is shaped as a regular tetrahedral.
- Repulsion between lone pair-bond pair of electrons effect the geometry of molecules.
Bond angle is the angle between two bonds of a molecule and it is determined based on the geometry.
[Bond angles:
Answer to Problem 4.25UKC
The geometry around the central atom in the given molecular model is tetrahedral.
Explanation of Solution
In the given molecular model,
Four atoms are bonded to the central atom. The angle around the central atom is around
Therefore,
The geometry of the given molecular model is tetrahedral.
(b)
Interpretation:
The geometry around the central atom in the given molecular model is to be determined.
Concept introduction:
Molecular shape can be predicted from the Lewis structure by using the valence-shell Electron-pair repulsion (VSEPR) model.
- Count the number of valence electron pairs (bond pairs and lone pairs).
- Assume that the valence electron pairs form a structure that allows them to be as far away from each other as possible.
- If there are only two bond pair electrons, the molecule is linear.
- If there are three bond pair electrons, the molecule is shaped like a trigonal planar.
- If there are four bond pair electrons, the molecule is shaped as a regular tetrahedral.
- Repulsion between lone pair-bond pair of electrons effect the geometry of molecules.
Bond angle is the angle between two bonds of a molecule and it is determined based on the geometry.
[Bond angles:
Answer to Problem 4.25UKC
The geometry around the central atom in the given molecular model is pyramidal.
Explanation of Solution
In the given molecular model,
Three atoms are bonded to the central atom. Three atoms are bonded to the central atom. The angle around the central atom is around
Therefore,
The geometry of the given molecular model is pyramidal.
(c)
Interpretation:
The geometry around the central atom in the given molecular model is to be determined.
Concept introduction:
Molecular shape can be predicted from the Lewis structure by using the valence-shell Electron-pair repulsion (VSEPR) model.
- Count the number of valence electron pairs (bond pairs and lone pairs).
- Assume that the valence electron pairs form a structure that allows them to be as far away from each other as possible.
- If there are only two bond pair electrons, the molecule is linear.
- If there are three bond pair electrons, the molecule is shaped like trigonal planar.
- If there are four bond pair electrons, the molecule is shaped as a regular tetrahedral.
- Repulsion between lone pair-bond pair of electrons effect the geometry of molecules.
Bond angle is the angle between two bonds of a molecule and it is determined based on the geometry.
[Bond angles:
Answer to Problem 4.25UKC
The geometry around the central atom in the given molecular model is trigonal planar.
Explanation of Solution
In the given molecular model,
Three atoms are bonded to the central atom. The angle around the central atom is around
Therefore,
The geometry of the given molecular model is trigonal planar.
Want to see more full solutions like this?
Chapter 4 Solutions
Fundamentals of General, Organic, and Biological Chemistry Plus Mastering Chemistry with Pearson eText -- Access Card Package (8th Edition)
- What structures are labeled 1 in the diagrams? What structure is labeled 2 in the diagrams? What is labeled 3 in both diagrams? What is labeled 4 in the diagrams? What is labeled 5 in the diagrams?arrow_forwardHow many oxygen atoms are in this molecular formula? 3H2 Oarrow_forwardConvert the following structural formulas into condensed structures.arrow_forward
- How does the bonding involved in a compound (nanoscopic interactions) influence the macroscopic physical properties that can be observed of the compound?arrow_forwardWhy is molecular polarity so important when discussing molecules? How can you determine where or not a molecule is (polar) or (non polar) with regard to there charge distribution?arrow_forwardMany molecules are polar, yet they do not form significanthydrogen bonds. What is so unusual about water thathydrogen bonding becomes possible?arrow_forward
- A compound with empirical formula C2H5O was found in a separate experiment to have a molar mass of approximately 90 g. What is the molecular formula of the compound?arrow_forwardUse Frost Circles to complete the molecular orbital diagram for cyclooctatetrane. Label the bonding, non bonding, and anti bonding MO’s. If the molecule is planar, would it be aromatic, antiaromatic, or nonaromatic? If the molecule is nonplanar, would it be aromatic, antiaromatic, or nonaromatic?arrow_forwardWhat are some of the ways that the features of carbon-to-carbon bonds influence the stability and 3-D structure of organic molecules?arrow_forward
- With respect to the above molecular outline, what chemical properties would you expect forthe (a) hydrophobic area and for the (b) hydrophilic area?arrow_forwardButadiene (right) is a colorless gas used to make synthetic rubber and many other compounds. (a) How many σ bonds and π bonds does the molecule have? (b) Are cis-trans arrangements about the double bonds possible? Explain.arrow_forwardHow many electrons are in the outer shell of each of the following atoms?arrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning


