
What phase change is shown in the accompanying molecular art? Is energy absorbed or released during the process?
Interpretation:
The phase change accompanying the molecular art and the process of reaction (exothermic and endothermic) has to be given.
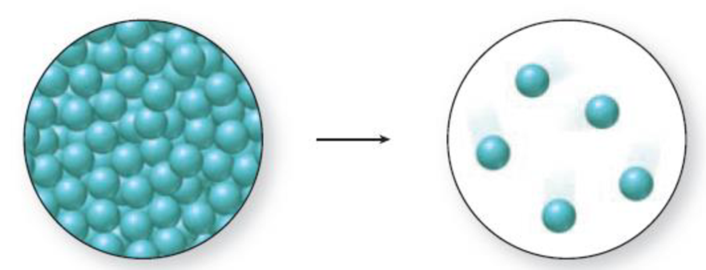
The given diagram is,
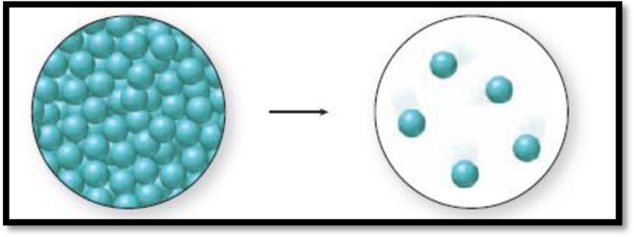
Figure 1
Concept introduction:
Endothermic Reactions:
The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. Few examples of endothermic process is photosynthesis, evaporating liquids, melting ice.
Exothermic reaction:
The exothermic reaction is the opposite of an endothermic reaction. It releases energy by light or heat to its surrounding. Few examples are neutralization, burning a substance, reactions of fuels, deposition of dry ice, respiration.
Vaporization:
Vaporization means conversion of a substance from the liquid phase to the gaseous phase.
Explanation of Solution
The given diagram is,
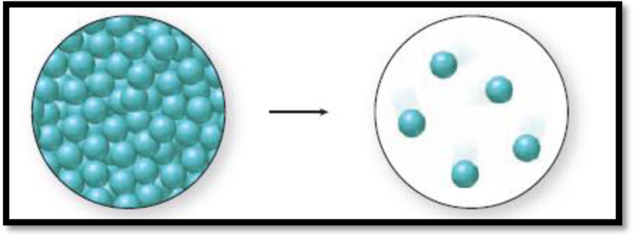
Figure 1
The change in phase can be identified by the distance between the spheres and the level of organization. A solid has closely packed spheres that are well organized, a liquid has closely packed but randomly arranged spheres, and a gas has randomly arranged spheres that are farther away from each other.
The diagram 1 represents a liquid and the diagram two represents a gas. Vaporization is the process of change from liquid to gas. Thus., the phase change accompanied in the figure 1 is a vaporization process and it is an endothermic process because liquid molecules absorb energy to go from liquid state to gaseous state.
Want to see more full solutions like this?
Chapter 4 Solutions
Principles of General, Organic, Biological Chemistry
- If 10. J of heat is applied to 5.0-g samples of each of the sub stances listed in Table 10.1, which substance’s temperature will increase the most? Which substance’s temperature wilt increase the least?arrow_forwardA representation of liquid water is shown below. Which of the three representations that follow best describes the water after it has boiled into steam?arrow_forwardThe amount of heat required to melt 2 lbs of ice is twice the amount of heat required to melt 1 lb of ice. Is this observation a macroscopic or microscopic description of chemical behavior? Explain your answer.arrow_forward
- Heat is added to boiling water. Explain why the temperature of the boiling water does not change. What does change?arrow_forwardif chart B represents the cooling of a 14.0 g of a substance at a constant rate, determine the amount of energy lost during the cooling of the liquid phasearrow_forwardEthyl chloride is sold as a liquid (see photo) under pressurefor use as a local skin anesthetic. Ethyl chloride boils at 12 °Cat atmospheric pressure. When the liquid is sprayed ontothe skin, it boils off, cooling and numbing the skin as itvaporizes. (a) What changes of state are involved in this useof ethyl chloride?arrow_forward
- Which phase can be described as "molecules roll around one another"arrow_forwardA total of 128 cal of heat is added to 7.25 g of ice at -45.0 degrees Celsius. What is the final temperature of the water?arrow_forwardA professor has a beaker full of pure water. She heats the water to it's boiling point of 100°C. Next, she adds consecutive allotments of a compound to the water and monitors the temperature of the water. What direction will the temperature change be?arrow_forward
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College DivChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College DivChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





