
Concept explainers
Name each
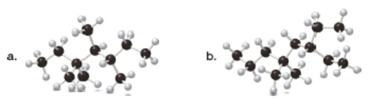
(a)
Interpretation: The nomenclature of given alkane is to be stated and each carbon as
Concept introduction: The aliphatic hydrocarbons that have only
Answer to Problem 4.34P
The IUPAC name of given alkane is
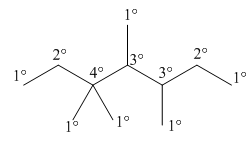
Figure 6
Explanation of Solution
The given ball and stick model of alkane is,
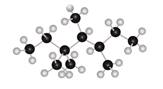
Figure 1
In ball-and-stick model, each colored ball represents a specific atom and each stick represents a bond. In this model, each black ball represents
Thus, the skeletal structure of given alkane is,
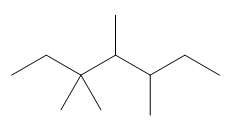
Figure 2
One should follow the given four steps to give the IUPAC name of a compound. The first step is naming of longest parent chain.
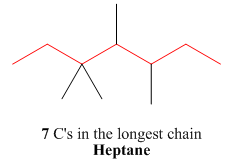
Figure 3
The second step is numbering of chain.
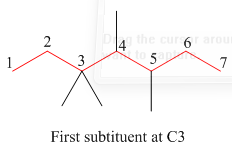
Figure 4
The third step is naming and numbering of substituents.
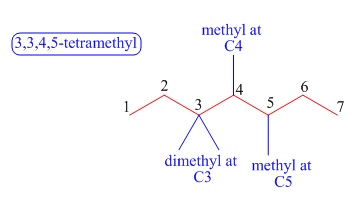
Figure 5
The fourth step is combining of all parts.
Thus, the IUPAC name of given alkane is
The aliphatic hydrocarbons that have only
Thus, each carbon as

Figure 6
The IUPAC name of given alkane is
(b)
Interpretation: The nomenclature of given alkane is to be stated and each carbon as
Concept introduction: The aliphatic hydrocarbons that have only
Answer to Problem 4.34P
The IUPAC name of given alkane is
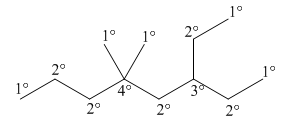
Figure 12
Explanation of Solution
The given ball and stick model of alkane is,
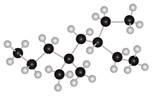
Figure 7
In ball-and-stick model, each colored ball represents a specific atom and each stick represents a bond. In this model, each black ball represents
Thus, the skeletal structure of given alkane is,
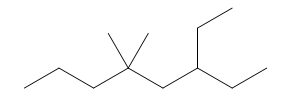
Figure 8
One should follow the given four steps to give the IUPAC name of a compound. The first step is naming of longest parent chain.
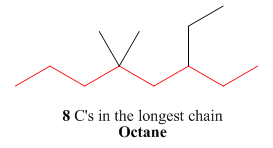
Figure 9
The second step is numbering of chain.
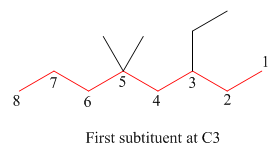
Figure 10
The third step is naming and numbering of substituents.
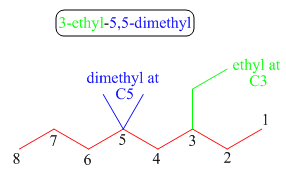
Figure 11
The fourth step is combining of all parts.
Thus, the IUPAC name of given alkane is
The aliphatic hydrocarbons that have only
Thus, each carbon as

Figure 12
The IUPAC name of given alkane is
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Essential Organic Chemistry (3rd Edition)
Organic Chemistry (8th Edition)
Chemistry
Organic Chemistry
General, Organic, & Biological Chemistry
- Write a condensed structural formula, such as CH3CH3, and describe the molecular geometry at each carbon atom. (a) propane. (b) 1-butanol. (c) ethyl propyl ether. (d) cis -4-bromo-2-heptene. (e) 2, 2, 3-trimethylhexane. (f) formaldehydearrow_forward1. What is the alkane product from the reaction of C7H13COONa and NaOH? a. pentane b. hexane c. butane d. heptane 2. What is the resulting alkane if we have C5H11F and a C5H11F as reactants in Wurtz synthesis? a. hexane b. octane c. nonane d. decane 3.What is the resulting alkane if we have 2C2H5Cl as reactants in Wurtz Synthesis reaction? a. ethane b. butane c. hexane d. octanearrow_forwardDraw structures that fit each description and name the functional group in each molecule: (a) two constitutional isomers with molecular formula C5H10O that contain different functional groups; (b) two constitutional isomers with molecular formula C6H10O that contain the same functional group.arrow_forward
- A2 1. An alkyne with molecular formula C5H10 2. A ketone with molecular formula C4H8O 3. A ketone with molecular formula C3H8O 4. An alkene with molecular formula C5H8 5. An alkene with molecular formula C5H10 6. An aldehyde with molecular formula C2H4O 7. An aldehyde with molecular formula CH4O 8. A saturated hydrocarbon with molecular formula C6H14arrow_forwardShow how to convert 1- Butene into these compounds. a. Butane b. 2- Butanol c. 2- Bromobutane d. 1,2- Dibromobutanearrow_forward1. The following are isomers of each other I. pentane II. 2,2,-dimethylpropane III. 2-methylbutaneIV. 2,3-dimethylhexane A. Alkanes are nonpolar. B. Alkanes are non-flammable. C. Every carbon in an alkane has four bonds. D. Alkanes contain only C and H atoms. E. Alkanes do not contain carbon-carbon double or triple bonds. 1b. Which of these is a weak base? A. NaOH B. H3PO4 C. CH3COOH D. NaHCO3 E. NH4NO3 1c. Which of these pairs are formed when NaHCO3 (baking soda) is dissolved in water? NaHCO3 → ______ + ______. A. NaOH + CO2 B. Na+ +HCO3- C. NaH+ +CO3- D. Na + HCO3 E. NaH- + CO3+ 1D. Which of these statements about water is(are) incorrect? A. It has a pH of 7.0 at ambient temperatures. B. It contains no ions. C. It is neutral. D. It can act as a proton donor. E. It can act as a proton acceptor.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax


