
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.68PAE
4.68 The pictures below show a molecular-scale view of a
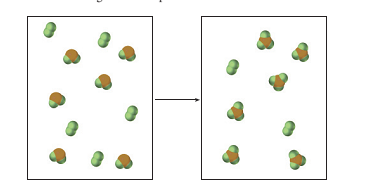
Was there a limiting reactant in this reaction? If so, what was it? Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 4 Solutions
Chemistry for Engineering Students
Ch. 4 - Describe the chemical composition of gasoline.Ch. 4 - Write balanced chemical equations for the...Ch. 4 - Prob. 3COCh. 4 - Calculate the amounts of reactants needed in a...Ch. 4 - Prob. 5COCh. 4 - Prob. 6COCh. 4 - Prob. 7COCh. 4 - 4.1 List at least two factors that make it...Ch. 4 - 4.2 What is an alkane?Ch. 4 - 4.3 Explain the difference between complete and...
Ch. 4 - 4.4 Automobile exhaust often contains traces of...Ch. 4 - 4.5 Methane, ethane, and propane are also...Ch. 4 - 4.6 Use the web to research prices of gasoline at...Ch. 4 - For the following reactions, write the ratios that...Ch. 4 - 4.8 In an experiment carried out at very low...Ch. 4 - 4.9 Sulfur, S8, combines with oxygen at elevated...Ch. 4 - 4.10 How many moles of oxygen can be obtained by...Ch. 4 - 4.11 MTBE, C5H12O, is one of the additives that...Ch. 4 - 4.12 In petroleum refining, hydrocarbons are often...Ch. 4 - 4.13 For the following reactions, determine the...Ch. 4 - 4.14 The combustion of liquid chloroethylene,...Ch. 4 - 4.15 What mass of the unknown compound is formed...Ch. 4 - 4.16 Many metals react with halogens to give metal...Ch. 4 - 4.17 Phosgene is a highly toxic gas that has been...Ch. 4 - Prob. 4.18PAECh. 4 - 4.19 How many metric tons of carbon are required...Ch. 4 - 4.20 Assuming a charcoal briquette is composed...Ch. 4 - 4.21 Ammonium nitrate, NH4NO3, will decompose...Ch. 4 - 4.22 Generally, an excess of O2 is needed for the...Ch. 4 - 4.23 In the reaction of arsenic with bromine,...Ch. 4 - 4.24 Ammonia gas can be prepared by the reaction...Ch. 4 - 4.25 When octane is combusted with inadequate...Ch. 4 - 4.26 The equation for one of the reactions in the...Ch. 4 - 4.27 Copper reacts with sulfuric acid according to...Ch. 4 - 4.28 One of the steps in the manufacture of nitric...Ch. 4 - 4.29 When Al(OH)3 reacts with sulfuric acid, the...Ch. 4 - 4.30 Copper reacts with nitric acid via the...Ch. 4 - 4.31 How much HNO3 can be formed in the following...Ch. 4 - 4.32 Hydrogen and oxygen are reacted and the water...Ch. 4 - 4.33 Silicon carbide, an abrasive, is made by the...Ch. 4 - Prob. 4.34PAECh. 4 - Prob. 4.35PAECh. 4 - 4.36 Sometimes students in chemistry labs...Ch. 4 - 4.37 The theoretical yield and the actual yield...Ch. 4 - 4.38 A reaction that produced 4.8 mg of taxol, an...Ch. 4 - Methanol, CH3OH, is used in racing cars because it...Ch. 4 - 4.40 When iron and steam react at high...Ch. 4 - 4.41 The percentage yield of the following...Ch. 4 - 4.42 Sulfur hexafluoride is a very stable gas...Ch. 4 - 4.43 Magnesium nitride forms in a side reaction...Ch. 4 - 4.44 Industrial production of hydrogen gas uses...Ch. 4 - 4.45 If 21 g of H2S is mixed with 38 g of O2 and...Ch. 4 - 4.46 A mixture of 10.0 g of NO and 14.0 g of NO2...Ch. 4 - 4.47 Silicon carbide is, an abrasive used in the...Ch. 4 - 4.48 Elemental phosphorous is used in the...Ch. 4 - 4.49 Small quantities of hydrogen gas can be...Ch. 4 - Prob. 4.50PAECh. 4 - 4.51 What is the role of an indicator in a...Ch. 4 - 4.52 What volume of 0.812 M HCl, in milliliters,...Ch. 4 - Prob. 4.53PAECh. 4 - Prob. 4.54PAECh. 4 - Hydrazine, N2H4, is a weak base and can react with...Ch. 4 - Prob. 4.56PAECh. 4 - Prob. 4.57PAECh. 4 - Prob. 4.58PAECh. 4 - 4.59 Aluminum dissolves in HCI according to the...Ch. 4 - 4.60 Why are fuel additives used?Ch. 4 - 4.61 What is actually measured by the octane...Ch. 4 - Prob. 4.62PAECh. 4 - Prob. 4.63PAECh. 4 - 4.64 Using the web, find information about the...Ch. 4 - 4.65 Using the web, find out how lead “poisons”...Ch. 4 - 4.66 If 3.4 mol Al is mixed with 1.5 times as many...Ch. 4 - 4.67 If 8.4 moles of disilane, Si2H6, are combined...Ch. 4 - 4.68 The pictures below show a molecular-scale...Ch. 4 - 4.69 The pictures below show a molecular-scale...Ch. 4 - 4.70 The particulate scale drawing shown depicts...Ch. 4 - 4.71 The particulate scale drawing shown depict...Ch. 4 - 4.72 The picture shown depicts the species present...Ch. 4 - Prob. 4.73PAECh. 4 - Prob. 4.74PAECh. 4 - Prob. 4.75PAECh. 4 - Prob. 4.76PAECh. 4 - You have 0.954 g of an unknown acid, H2A, which...Ch. 4 - Prob. 4.78PAECh. 4 - 4.79 Phosphoric add (H3PO4) is important in the...Ch. 4 - 4.80 The reaction shown below is used to destroy...Ch. 4 - Prob. 4.81PAECh. 4 - One way of determining blood alcohol levels is by...Ch. 4 - Prob. 4.83PAECh. 4 - 4.84 Aluminum chloride (AlCl3) is used as a...Ch. 4 - 4.85 In the cold vulcanization of rubber, disulfur...Ch. 4 - Prob. 4.86PAECh. 4 - Prob. 4.87PAECh. 4 - 4.88 A quality control technician needs to...Ch. 4 - Prob. 4.89PAECh. 4 - 4.90 Iron metal can be refined (rom the mineral...Ch. 4 - Prob. 4.91PAECh. 4 - Prob. 4.92PAECh. 4 - 4.93 A mixture of methane (CH4) and propane (C3H8)...Ch. 4 - Prob. 4.94PAECh. 4 - Prob. 4.95PAECh. 4 - Prob. 4.96PAECh. 4 - Prob. 4.97PAECh. 4 - Prob. 4.98PAECh. 4 - Prob. 4.99PAECh. 4 - Prob. 4.100PAECh. 4 - Prob. 4.101PAECh. 4 - Prob. 4.102PAECh. 4 - Prob. 4.103PAECh. 4 - 4.104 When 2.750 g of the oxide Pb3O4 is heated to...Ch. 4 - Prob. 4.105PAECh. 4 - 4.106 An ore sample with a mass of 670 kg contains...Ch. 4 - 4.107 Existing stockpiles of the refrigerant...Ch. 4 - 4.108 Elemental analysis is sometimes carried out...Ch. 4 - Prob. 4.109PAECh. 4 - 4.110 Write the balanced chemical equation lot the...Ch. 4 - 4.111 Aluminum metal reacts with sulfuric acid to...Ch. 4 - 4.112 A metallurgical firm wishes to dispose of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4.69 The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce methanol, CH3OH. The box on the left represents the reactants at the instant of mixing, and the box on the right shows what is left once the reaction has gone to completion. Was there a limiting reactant in this reaction? If so, what was it? Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.arrow_forwardSilicon is produced for the chemical and electronics industries by the following reactions. Give the balanced equation for each reaction. a. SiO2(s)+C(s)arefurnaceElectricSi(s)+CO(g) b. Liquid silicon tetrachloride is reacted with very pure solid magnesium, producing solid silicon and solid magnesium chloride. c. Na2SiF6(s) + Na(s) Si(s) + NaF(s)arrow_forwardPropane, C3H8, is the fuel of choice in a gas barbecue. When burning, the balanced equation is C3H8+5O23CO2+4H2O a What is the limiting reactant in cooking with a gas grill? b If the grill will not light and you know that you have an ample flow of propane to the burner, what is the limiting reactant? c When using a gas grill you can sometimes turn the gas up to the point at which the flame becomes yellow and smokey. In terms of the chemical reaction, what is happening?arrow_forward
- 4.84 Aluminum chloride (AlCl3) is used as a catalyst in the production of polyisobutylene, which is used in automobile tires. Scrap aluminum metal reacts with chlorine gas (Cl2) to produce AlCl3. Suppose that 2.70 g of Al and 7.10 g of Cl2 are mixed. What is the maximum mass of AlCl3 that could be formed?arrow_forward3.75 The following pictures show a molecular-scale view of a chemical reaction between the compounds AB2 and B2. (A atoms are shown in blue and B atoms in white). The box on the left represents the reactants at the instant of mixing, and the box on the right shows what is left once the reac- tion has gone to completion. Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.arrow_forwardBacterial digestion is an economical method of sewage treatment. The reaction is an intermediate step in the conversion of the nitrogen in organic compounds into nitrate ions. What mass of bacterial tissue is produced in a treatment plant for every 1.0 104 kg of wastewater containing 3.0% NH4+ ions by mass? Assume that 95% of the ammonium ions are consumed by the bacteria.arrow_forward
- 4-25 In the chemical test for arsenic, the gas arsme, AsH3, is prepared. When arsine is decomposed by heating, arsenic metal deposits as a mirror-like coating on the surface of a glass container and hydrogen gas, H2, is given off. Write a balanced equation for the decomposition of arsine.arrow_forward4.106 An ore sample with a mass of 670 kg contains 27.7% magnesium carbonate, MgCO3. If all of the magnesium carbonate in this ore sample is decomposed to form carbon dioxide, describe how to determine what mass of CO2 is evolved during the process.arrow_forwardDisulfur dichloride, S2Cl2, is used to vulcanize rubber. It can be made by treating molten sulfur with gaseous chlorine: S8(l) + 4 Cl2(g) 4 S2Cl3(l) Starting with a mixture of 32.0 g of sulfur and 71.0 g of Cl2, (a) Which is the limiting reactant? (b) What is the theoretical yield of S2Cl2? (c) What mass of the excess reactant remains when the reaction is completed?arrow_forward
- 4.8 In an experiment carried out at very low pressure, 13x1015 molecules of H2 are reacted with acetylene, C2H2, to form ethane, C2H6, on the surface of a catalyst. Write a balanced chemical equation for this reaction. How many molecules of acetylene are consumed?arrow_forwardZinc acetate is sometimes prescribed by physicians for the treatment of Wilsons disease, which is a genetically caused condition wherein copper accumulates to toxic levels in the body. If you were to analyze a sample of zinc acetate and find that it contains 3.33 1023 acetate ions, how many grams of zinc acetate must be present in the sample?arrow_forward3.122 What type of reasoning were we using when we developed the equation for dilution, MiVi=MfVf ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY