
Concept explainers
(a)
Interpretation:
To show the net result is the decomposition of water
Concept Introduction:
Heat energy required to raise the temperature of 1g of substance by 1K.Energy gained or lost can be calculated using the below equation.
Where, q= energy gained or lost for a given mass of substance (m), C =specific heat capacity,
The standard molar enthalpy of formation is the enthalpy change
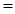
(a)
Explanation of Solution
To show the net result is the decomposition of water.
Equation
Equation
Equation
(b)
Interpretation:
If
Concept Introduction:
Heat energy required to raise the temperature of 1g of substance by 1K.Energy gained or lost can be calculated using the below equation.
Where, q= energy gained or lost for a given mass of substance (m), C =specific heat capacity,
The standard molar enthalpy of formation is the enthalpy change
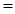
(b)
Explanation of Solution
The mass of
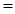
Substitute the values in,
The reaction is
(c)
Interpretation:
The enthalpy of reaction has to be calculated.
Concept Introduction:
Heat energy required to raise the temperature of 1g of substance by 1K.Energy gained or lost can be calculated using the below equation.
Where, q= energy gained or lost for a given mass of substance (m), C =specific heat capacity,
The standard molar enthalpy of formation is the enthalpy change
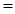
(c)
Explanation of Solution
Given reaction is:
Substitute the values in 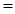
Substitute the values in 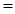
(d)
Interpretation:
Comment on the feasibility of using such a series of reaction to produce
Concept Introduction:
Heat energy required to raise the temperature of 1g of substance by 1K.Energy gained or lost can be calculated using the below equation.
Where, q= energy gained or lost for a given mass of substance (m), C =specific heat capacity,
The standard molar enthalpy of formation is the enthalpy change
(d)
Explanation of Solution
Since the enthalpy is positive the reaction is not feasible
Want to see more full solutions like this?
Chapter 5 Solutions
Chemistry & Chemical Reactivity
- Water gas, a mixture of carbon monoxide and hydrogen, is produced by treating carbon (in the form of coke or coal) with steam at high temperatures. (See Study Question 83.) C(s) + H2O(g) CO(g) + H2(g) Not all of the carbon available is converted to water gas since some is burned to provide the heat for the endothermic reaction of carbon and water. What mass of carbon must be burned (to CO2 gas) to provide the energy to convert 1.00 kg of carbon to water gas?arrow_forwardIn a bomb calorimeter, the reaction vessel is surrounded by water that must be added for each experiment. Since the amount of water is not constant from experiment to experiment, the mass of water must be measured in each case. The heat capacity of the calorimeter is broken down into two parts: the water and the calorimeter components. If a calorimeter contains 1.00 kg water and has a total heat capacity of 10.84 kJ/C, what is the heat capacity of the calorimeter components?arrow_forwardInsoluble PbBr2(s) precipitates when solutions of Pb(NO3)2(aq) and NaBr(aq) are mixed. Pb(NO3)2(aq) + 2 NaBr(aq) PbBr2(s) + 2 NaNO3(aq) rH = ? To measure the enthalpy change, 200. mL. of 0.75 M Pb(NO3)2(aq) and 200. mL of 1.5 M NaBr(aq) are mixed in a coffee-cup calorimeter. The temperature of the mixture rises by 2.44 C. Calculate the enthalpy change for the precipitation of PbBr2(s), in kJ/mol. (Assume the density of the solution is 1.0 g/mL., and its specific heat capacity is 4.2 J/g K.)arrow_forward
- Combustion of table sugar produces CO2(g) and H2O( l). When 1.46 g table sugar is combusted in a constant-volume (bomb) calorimeter, 24.00 kJ of heat is liberated. a. Assuming that table sugar is pure sucrose, C12H22O11 (s), write the balanced equation for the combustion reaction. b. Calculate E in kJ/mol C12H22O11 for the combustion reaction of sucrose.arrow_forwardThe decomposition of ozone, O3, to oxygen, O2, is an exothermic reaction. What is the sign of q? If you were to touch a flask in which ozone is decomposing to oxygen, would you expect the flask to feel warm or cool?arrow_forwardWhat mass of acetylene, C2H2(g), must be burned to produce 3420 kJ of heat, given that its enthalpy of combustion is 1301 kJ/mol? Compare this with the answer to Exercise 5.91 and determine which substance produces more heat per gram.arrow_forward
- The complete combustion of acetylene, C2H2(g), produces 1300. kJ of energy per mole of acetylene consumed. How many grams of acetylene must be burned to produce enough heat to raise the temperature of 1.00 gal water by 10.0c if the process is 80.0% efficient? Assume the density of water is 1.00 g/cm3arrow_forwardAn industrial process for manufacturing sulfuric acid, H2SO4, uses hydrogen sulfide, H2S, from the purification of natural gas. In the first step of this process, the hydrogen sulfide is burned to obtain sulfur dioxide, SO2. 2H2S(g)+3O2(g)2H2O(l)+2SO2(g);H=1124kJ The density of sulfur dioxide at 25C and 1.00 atm is 2.62 g/L, and the molar heat capacity is 30.2 J/(mol C). (a) How much heat would be evolved in producing 1.00 L of SO2 at 25C and 1.00 atm? (b) Suppose heat from this reaction is used to heat 1.00 L of the SO2 from 25C to 500C for its use in the next step of the process. What percentage of the heat evolved is required for this?arrow_forwardHypothetical elements A2 and B2 react according to the following equation, forming the compound AB. A2(aq)+B2(aq)2AB(aq);H=+271kJ/mol If solutions A2(aq) and B2(aq), starting at the same temperature, are mixed in a coffee-cup calorimeter, the reaction that occurs is a exothermic, and the temperature of the resulting solution rises. b endothermic, and the temperature of the resulting solution rises. c endothermic, and the temperature of the resulting solution falls. d exothermic, and the temperature of the resulting solution falls. e exothermic or endothermic, depending on the original and final temperatures.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





