
Concept explainers
Biofuels
A lot of energy is locked up in the
Corn, soy, sugarcane, and other food crops are rich in oils, starches, and sugars that can be easily converted to biofuels. The starch in corn kernels, for example, can be enzymatically broken down to glucose, which is fermented to ethanol by bacteria or yeast. However, growing food crops for biofuel production typically requires a lot of energy (in the form of fossil fuels) and it damages the environment. Making biofuels from other plant matter such as weeds or agricultural waste requires additional steps, because these materials contain a higher proportion of cellulose. Breaking down this tough carbohydrate to its glucose monomers adds cost to the biofuel product.
In 2006, David Tilman and his colleagues published the results of a 10-year study comparing the net energy output of various biofuels. The researchers made biofuel from a mixture of native perennial grasses grown without irrigation, fertilizer, pesticides, or herbicides, in sandy soil that was so depleted by intensive agriculture that it had been abandoned. The energy content of this biofuel and the energy it took to produce it were measured and compared with that of biofuels made from food crops (Figure 5.16).
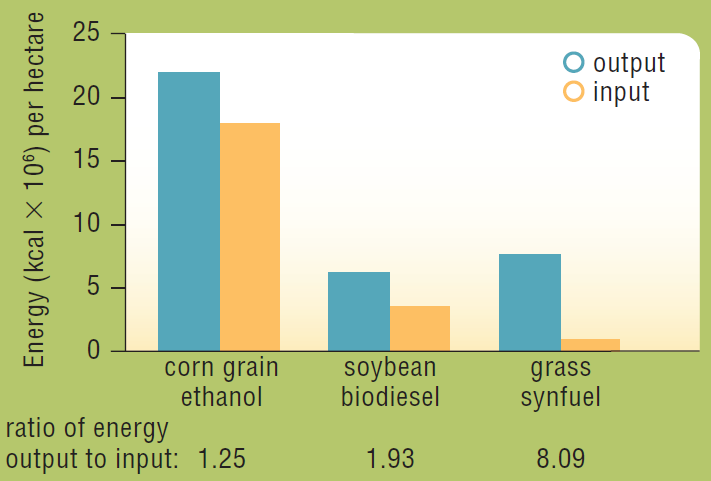
About how much energy did ethanol produced from one hectare of corn yield? How much energy did it take to grow and produce that ethanol?
To determine: How much energy did the ethanol produced from one hectare of corn yield and how much energy did it took to grow the corn.
Concept introduction:The plants and other organic material other than fossils can also be used as a source of energy. The oils, gases, and alcohols made from these materials are called biofuels. Corn and other food crops are rich in oils, starches, and sugars that can be easily converted into biofuels.
Explanation of Solution
The researcher D and his colleagues studied for 10 years and compared the net energy output of various biofuels. The researcher grew a mixture of native perennial grasses, corn, and soy. The grasses grew without irrigation, fertilizer, and pesticides in sandy soil. The usable energy in biofuel (grasses, corn, and soy) is measured along with the energy it took to grow.
Refer to Fig. 6.1 “Energy inputs and outputs of biofuels made from three different crops” in the question. The graph plot shows the energy per hectare versus the ratio of energy output to input of the three different biofuels.
The graph shows the ethanol obtained from one hectare of corn produced
The ethanol from one hectare of corn produced approximately 23 × 106 kcal. It took approximately 18 × 106 kcal energy to grow the corn that was used to make the ethanol.
Want to see more full solutions like this?
Chapter 5 Solutions
Biology Today and Tomorrow without Physiology (MindTap Course List)
- Biofuels A lot of energy is locked up in the chemical bonds of molecules made by plants. That energy can fuel consumers, as when an animal cell powers ATP synthesis by aerobic respiration. It can also fuel our cars, which run on energy released by burning biofuels or fossil fuels. Both processes are fundamentally the same: They release energy by breaking the bonds of organic molecules. Both use oxygen to break those bonds, and both produce carbon dioxide. Unlike fossil fuels, biofuels are a renewable source of energy: We can always make more of them simply by growing more plants. Also unlike fossil fuels, biofuels do not contribute to global climate change, because growing plant matter for fuel recycles carbon that is already in the atmosphere. Corn, soy, sugarcane, and other food crops are rich in oils, starches, and sugars that can be easily converted to biofuels. The starch in corn kernels, for example, can be enzymatically broken down to glucose, which is fermented to ethanol by bacteria or yeast. However, growing food crops for biofuel production typically requires a lot of energy (in the form of fossil fuels) and it damages the environment. Making biofuels from other plant matter such as weeds or agricultural waste requires additional steps, because these materials contain a higher proportion of cellulose. Breaking down this tough carbohydrate to its glucose monomers adds cost to the biofuel product. In 2006, David Tilman and his colleagues published the results of a 10-year study comparing the net energy output of various biofuels. The researchers made biofuel from a mixture of native perennial grasses grown without irrigation, fertilizer, pesticides, or herbicides, in sandy soil that was so depleted by intensive agriculture that it had been abandoned. The energy content of this biofuel and the energy it took to produce it were measured and compared with that of biofuels made from food crops (Figure 5.16). The production of which biofuel was most efficient (which had the highest ratio of energy output to energy input)?arrow_forwardAs the sun shines on Earth, plants use its energy to complete the process of photosynthesis. Energy is transformed during the process into chemical energy in the form of sugars, such as carbohydrates. However, the energy available at top consumers is much less than that produced by autotrophs. Why is this so?arrow_forwardLight is a form of electromagnetic energy. The electromagnetic spectrum is made of various forms of electromagnetic energy, such as x-rays, UV, and microwaves. Visible light is used in photosynthesis. What is visible light? How are energy and wavelength related to each other? The overall goal of photosynthesis is to make harness the energy of the sun and store it in the chemical bonds of “food.” In your own words describe the goals of the two sets of reactions of photosynthesis: a.The goal of the light reactions b. The goal of the Calvin cyclearrow_forward
- Fill in the blanks. Blanks with the same letter are the same molecule. Molecules to use: ATP, CO2, H2O, O2, sugars. Cellular respiration requires (a)______________ and oxidizes (b)____________ to release free energy and regenerate (c)____________, with (d)____________ and (e)___________ released as byproducts. Photosynthesis uses light energy to split (d)___________ and to reduce (e)___________ to build (b)__________ for storing free energy, with (a)___________ and (d)___________ released as byproducts.arrow_forwardWRITE ABOUT A THEME: ENERGY AND MATTER Life issolar powered. Almost all the producers of the biospheredepend on energy from the sun to produce the organicmolecules that supply the energy and carbon skeletonsneeded for life. In a short essay (100–150 words), describehow the process of photosynthesis in the chloroplasts ofplants transforms the energy of sunlight into the chemicalenergy of sugar molecules.arrow_forwardThe words exergonic and endergonic can be used to describe many reactions including those essential to life. Photosynthesis is an _reactionarrow_forward
- Autotrophs synthesis food for the living world. Justify this statement in one sentence only interconnecting autotrophs and heterotrophs.arrow_forwardThe connection between producers and consumers is that only: A) heterotrophs can perform photosynthesis to produce energy for all life B) autotrophs can use sun energy to u to produce energy for all organisms C) producers use cellular respiration to produce energy for all life D) heterotrophs can perform cellular respiration producing oxygen for all organismsarrow_forwardChemical bond energy: Is the energy used by photosynthesis for the fixation of carbon Is generated as usable energy for the cell Is generated by processes in the chloroplast and mitochondria All of the abovearrow_forward
- What happens to a molecule when it is oxidized? Electrons are gained Electrons are lost Electrons are converted into neutrons Electrons are converted into protons Electrons combine with a proton to produce a protoelectronarrow_forwardHello sir/miss, the question and answer are provided below. Is the answer to the question correct? Explain how the carbon cycle is related to energy flow in ecosystems. The carbon cycle is related to energy flow in ecosystems because they both involve photosynthesis. In the carbon cycle, carbon dioxide plays a role in plants and microorganisms using photosynthesis. The energy flow in ecosystems absorbs a small part of the Sun’s radiant energy (0.023%) by living organisms for photosynthesis.arrow_forwardPhotosynthesis is the process of breaking down glucose to produce ATP to fuel activities that occur in living things. producing glucose by fixing atmospheric carbon to broken down water molecules using energy from the sun. catalyzing chemical reactions at a cellular level to speed up bodily functions.arrow_forward
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax


