
Biochemistry
6th Edition
ISBN: 9781305577206
Author: Reginald H. Garrett, Charles M. Grisham
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 3P
Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book.
Solving the Sequence of an Oligopeptide From Sequence Analysis Data Amino acid analysis of a decapeptide revealed the presence of the following products:
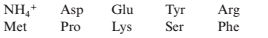
The following facts were observed:
- Neither car boxy peptidase A nor B treatment of the- decapeptide had any effect.
- Trypsin treatment yielded two tetrapcptides and free Lys.
- Clostripain treatment yielded a tetrapcptide and a hexapeptidc.
- Cyanogen bromide treatment yielded an octapeptide and a dipeptide of sequence NP (using the one-letter codes).
- Chymotrypsin treatment yielded two tripeptides and a telrapeptide. The N-terminal chymotryptic peptide had a net charge of — 1 at neutral pi I and a net charge of —3 al pH 12.
- One cycle of Ed man degradation gave the PTH derivative
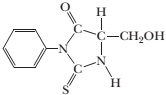
What is the ammo acid sequence of this decapeptide?
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 5 Solutions
Biochemistry
Ch. 5 - Answers to all problems are at the end of this...Ch. 5 - Answers to all problems are at the end of this...Ch. 5 - Answers to all problems are at the end of this...Ch. 5 - Answers to all problems are at the end of this...Ch. 5 - Answers to all problems are at the end of this...Ch. 5 - Answers to all problems are at the end of this...Ch. 5 - Answers to all problems are at the end of this...Ch. 5 - Answers to all problems are at the end of this...Ch. 5 - Answers to all problems are at the end of this...Ch. 5 - Answers to all problems are at the end of this...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Interpreting Kinetics Experiments from Graphical Patterns The following graphical patterns obtained from kinetic experiments have several possible interpretations depending on the nature of the experiment and the variables being plotted. Give at least two possibilities for each.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. (Research Problem) The Nature and Roles of Linear Motifs in Proteins In addition to domains and modules, there are other significant sequence patterns in proteins—known as linear motifs—that are associated with a particular function. Consult the biochemical literature to answer the following questions: 1. What are linear motifs? 2. How are they different from domains?. 3. What are their functions? 4. How can they be characterized? 5. There are several papers that are good starting points for this problem. Neduva, V., and Russell, R., 2005. Linear motifs: evolutionary interaction switches. FEBS Letters 579:3342-3345. Gibson, T., 2009. Cell regulation: determined to signal discrete cooperation. Trends in Biochemical Sciences 34:471-482. Diella, K. Haslam, N., Chica., C. et aL, 2009. Understanding eukaryotic linear motifs and their role in cell signaling and regulation. Frontiers of Bioscience 13:6580-6603.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Evaluation of -Helices in Proteins The hem agglutinin protein in influenza virus contains a remarkably long -helix, with 53 residues. How long is this -helix (in nm)? How many turns does this helix have? The typical residue in an -helix is involved in two H bonds. How many H bonds are present in this helix?arrow_forward
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Solving the Sequence of an Oligopeptide From Sequence Analysis Data Amino acid analysis of ail oligopeptide seven residues long gave The following fads were observed: a. Trypsin treatment had no apparent effect. b. The phenylthiohydantoin released by Lid mini degradation was c. Brief chymotrypsin treatment yielded several products, including a dipeptide and a tetrapeptide. The amino acid composition of the tetrapeptide was Leu, Lyi. and Met. d. Cyanogen bromide treatment yielded a dipeptide, a tetrapeptide, and free Lys. What is the amino acid sequence of this heptapeptide?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. To fully appreciate the elements of secondary structure in proteins, it is useful to have a practical sense of their structures. On a piece of paper, draw a simple but large zigzag pattern to represent a -strand. Then fill in the structure, drawing the locations of the moms of the chain on this zigzag pattern. Then draw a simple, large coil on a piece of paper to represent an -helix. Then fill in the structure, drawing the backbone atoms in the correction locations along the coil and indicating the locations of the R groups in your drawing.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. The Role of Proline Residues in -Turns Pro is the amino acid least commonly found in «-helices but most commonly found in -turns. Discuss the reasons for this behavior.arrow_forward
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Calculating Tms and Separating DNA Molecules That Differ in G:C Content At 0.2 M Na+, the melting temperature of double-stranded DNA is given by the formula, Tm = 69.3 + 0 41 (% G + C). The DNAs from mice and rats have (G + C) contents of 44% and 40%, respectively. Calculate the Tms for these DNAs in 0.2 M NaCl. If samples of these DNAs were inadvertently mixed, how might they be separated from one another?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Solving the Sequence of an Oligopeptide From Sequence Analysis Data Amino acid analysis of an oligopeptide containing nine residues revealed the presence of the following amino adds: Arg Cys Gly Leu Met Pro Tyr Val The following was found: Carboxypeptidase A treatment yielded no free amino add. Edman analysis of the intact oligopeptide released c. Neither trypsin nor ehymotrypsin treatment of the nonapeptide released smaller fragments. However, combined trypsin and chymotrypsin treatment liberated free Arg. CNBr treatment of the eight-residue fragment left after combined trypsin and chymotrypsin action yielded a six-residue fragment containing Cys* Gly. Pro, Tyr, and Val and a dipeptide. Treatment of the six-residue fragment with -mercaptoethanol yielded two tripeptidcs. Brief Edman analysis of the tripeplide mixture yielded only Ρ�Ή-Cys. (The sequence of each tripeptide, as read from the N-terminal end, is alphabetical if the one-lelter designation for amino acids is used.) What is the amino acid sequence of this nonapeptide?arrow_forwardAnswers to all problems are at the end of this book Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Solving the Sequence of an Oligopeptide From Sequence Analysis Data Analysis of the blood of a catatonic football fan revealed large concentrations of a. psychologic octapeptide. Amino acid analysis of this oclapeplide gave the following results: 2 Ala lArg 1 Asp 1 Mel 2 Tyr I Val 1NH/ The following facts were observed: Partial acid hydrolysis of the octapeptide yielded a dipeptide of the structure Chymolrypsin treatment of the octapeplide yielded two tetrapeptides, each containing an alanine residue. Trypsin treatment of one of the tetrapeptides yielded two dipeptides. Cyanogen bromide treatment of another sample of the same tetrapeplide yielded a tripeplideand free Tyr. N-lerminal analysis of the other tetrapeptide gave Asn. What is the amino acid sequence of this oclapeplide?arrow_forward
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Consider the following peptide sequences: EANQIDEMLYNVQCS LTTLE DTVPW LG VHLDITVPL SWTWTLYVKL QQNWGGLWILTLVWFLM CNMKHGDSQCDERTYP YTREQSDGHIPKMNCDS AGPFGPDGPTIGPK Which of the preceding sequences would be likely to be found in each of the following: A parallel -sheet An antiparallel -sheet A tropocollagen molecule The helical portions of a protein found in your hairarrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Draw the Titration Curve for a Weak Acid and Determine its pKa from the Titration Curve When a 0.1 M solution of a weak acid was titrated with base, the following results were obtained: Plot the results of this titration and determine the pK a of the weak acid from your graph.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Use examples from the ActiveModel for Human GaleLtin-1 to describe the hydrophobic effect.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY