
An Introduction to Physical Science
14th Edition
ISBN: 9781305079137
Author: James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 4SA
An older type of thermostat used in furnace and heat pump control is shown in ● Fig. 5.21. The glass vial tilts back and forth so that electrical contacts are made via the mercury (an electrically
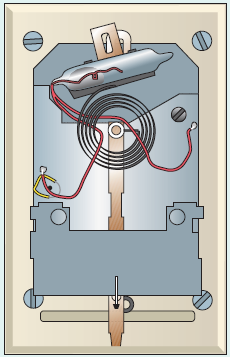
Figure 5.21 An Exposed View of a Thermostat See Short Answer Question 4.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
One easy way to reduce heating (and cooling) costs is to add extra insulation in the attic of a house. Suppose a single-story cubical house already had 15 cm of fiberglass insulation in the attic and in all the exterior surfaces. If you added an extra 8.0 cm of fiberglass to the attic, by what percentage would the heating cost of the house drop? Take the house to have dimensions 10 m by 15 m by 3.0m. Ignore air infiltration and heat loss through windowsand doors, and assume that the interior is uniformly at one temperature and the exterior is uniformly at another.
At what net rate does heat radiate from a 275m ^ 2 black roof on a night when the roof's temperature is 30.0 degree celcius and the surrounding temperature is 15.0 degree celcius. The emissivity of the roof is 0.900Your answer must have three significant figures and units of kw and a negative sign indicates heat loss.
On a hot day, the temperature of an 80,000-L swimming pool increases by 1.50ºC . What is the net heat transfer during this heating? Ignore any complications, such as loss of water by evaporation.
Chapter 5 Solutions
An Introduction to Physical Science
Ch. 5.1 - We talk about temperature, but what does it...Ch. 5.1 - Are there any limits on the lowest and highest...Ch. 5.1 - Show that a temperature of 40 is the same on both...Ch. 5.2 - Prob. 1PQCh. 5.2 - Most substances contract with decreasing...Ch. 5.3 - What is specific about specific heat?Ch. 5.3 - Prob. 2PQCh. 5.3 - Prob. 5.2CECh. 5.3 - How much heat must be removed from 0.20 kg of...Ch. 5.4 - What are the three methods of heat transfer?
Ch. 5.4 - Prob. 2PQCh. 5.5 - Prob. 1PQCh. 5.5 - Prob. 2PQCh. 5.6 - In the ideal gas law, pressure is directly...Ch. 5.6 - Prob. 2PQCh. 5.6 - Prob. 5.4CECh. 5.7 - Prob. 1PQCh. 5.7 - Prob. 2PQCh. 5 - Prob. AMCh. 5 - Prob. BMCh. 5 - Prob. CMCh. 5 - Prob. DMCh. 5 - Prob. EMCh. 5 - Prob. FMCh. 5 - Prob. GMCh. 5 - Prob. HMCh. 5 - Prob. IMCh. 5 - Prob. JMCh. 5 - Prob. KMCh. 5 - Prob. LMCh. 5 - Prob. MMCh. 5 - Prob. NMCh. 5 - Prob. OMCh. 5 - Prob. PMCh. 5 - Prob. QMCh. 5 - Prob. RMCh. 5 - Prob. SMCh. 5 - Prob. TMCh. 5 - Prob. UMCh. 5 - Prob. VMCh. 5 - Prob. WMCh. 5 - Prob. XMCh. 5 - Prob. YMCh. 5 - Prob. 1MCCh. 5 - Which unit of the following is smaller? (5.2) (a)...Ch. 5 - Prob. 3MCCh. 5 - Prob. 4MCCh. 5 - Prob. 5MCCh. 5 - Prob. 6MCCh. 5 - Prob. 7MCCh. 5 - Which of the following has a definite volume but...Ch. 5 - If the average kinetic energy of the molecules in...Ch. 5 - When we use the ideal gas law, the temperature...Ch. 5 - Prob. 11MCCh. 5 - Prob. 12MCCh. 5 - When a bimetallic strip is heated, it bends away...Ch. 5 - Prob. 2FIBCh. 5 - Prob. 3FIBCh. 5 - Prob. 4FIBCh. 5 - Prob. 5FIBCh. 5 - Prob. 6FIBCh. 5 - Prob. 7FIBCh. 5 - The ___ phase of matter has no definite shape, and...Ch. 5 - Prob. 9FIBCh. 5 - In the ideal gas law, pressure is ___ proportional...Ch. 5 - Prob. 11FIBCh. 5 - Prob. 12FIBCh. 5 - When the temperature changes during the day, which...Ch. 5 - Prob. 2SACh. 5 - The two common liquids used in liquid-in-glass...Ch. 5 - An older type of thermostat used in furnace and...Ch. 5 - Heat may be thought of as the middleman of energy....Ch. 5 - When one drinking glass is stuck inside another,...Ch. 5 - Prob. 7SACh. 5 - What does the specific heat of a substance tell...Ch. 5 - When eating a piece of hot apple pie, you may find...Ch. 5 - Prob. 10SACh. 5 - When you exhale outdoors on a cold day, you can...Ch. 5 - Compare the SI units of specific heat and latent...Ch. 5 - Give two examples each of good thermal conductors...Ch. 5 - Prob. 14SACh. 5 - Prob. 15SACh. 5 - Thermal underwear is made to fit loosely. ( Fig....Ch. 5 - What determines the phase of a substance?Ch. 5 - Give descriptions of a solid, a liquid, and a gas...Ch. 5 - Prob. 19SACh. 5 - How does the kinetic theory describe a gas?Ch. 5 - Prob. 21SACh. 5 - Prob. 22SACh. 5 - Prob. 23SACh. 5 - In terms of kinetic theory, explain why a...Ch. 5 - Prob. 25SACh. 5 - Prob. 26SACh. 5 - Prob. 27SACh. 5 - Prob. 28SACh. 5 - What can be said about the total entropy of the...Ch. 5 - Prob. 30SACh. 5 - Prob. 31SACh. 5 - Prob. 1VCCh. 5 - Prob. 1AYKCh. 5 - Prob. 2AYKCh. 5 - Prob. 3AYKCh. 5 - Prob. 4AYKCh. 5 - Prob. 5AYKCh. 5 - Prob. 6AYKCh. 5 - When you freeze ice cubes in a tray, there is a...Ch. 5 - Prob. 8AYKCh. 5 - Prob. 1ECh. 5 - Prob. 2ECh. 5 - Prob. 3ECh. 5 - Prob. 4ECh. 5 - Researchers in the Antarctic measure the...Ch. 5 - Prob. 6ECh. 5 - A college student produces about 100 kcal of heat...Ch. 5 - Prob. 8ECh. 5 - A pound of body fat stores an amount of chemical...Ch. 5 - Prob. 10ECh. 5 - On a brisk walk, a person burns about 325 Cal/h....Ch. 5 - Prob. 12ECh. 5 - How much heat in kcal must be added to 0.50 kg of...Ch. 5 - Prob. 14ECh. 5 - (a) How much energy is necessary to heat 1.0 kg of...Ch. 5 - Equal amounts of heat are added to equal masses of...Ch. 5 - How much heat is necessary to change 500 g of ice...Ch. 5 - A quantity of steam (300 g) at 110C is condensed,...Ch. 5 - Prob. 19ECh. 5 - A fire breaks out and increases the Kelvin...Ch. 5 - A cylinder of gas is at room temperature (20C)....Ch. 5 - A cylinder of gas at room temperature has a...Ch. 5 - A quantity of gas in a piston cylinder has a...Ch. 5 - If the gas in Exercise 23 is initially at room...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Calculate and plot the increase of cumulative kinematic heat (cooling) during the night of 16 hours, for a case with kinematic heat flux (km/s) of: a) -0.02 b) -0.05 c) -0.01 d) -0.04Carrow_forwardOn a hot dry day, evaporation from a lake has just enough heat transfer to balance the 1.00 kW/m2 of incoming heat from the Sun. What mass of water evaporates in 1.00 h from each square meter?arrow_forwardAt what rate is heat lost through a 1.7m x 1.4m rectangular glass windowpane that is 1.0cm thick when the inside temperature is 23.3 degrees Celsius and the outside temperature is 4.6 degrees Celsius?arrow_forward
- In what way does the glass act like a one-way valve for a conventional greenhouse? Does the atmosphere play the same role?arrow_forwardIs there more than one method of heat transfer? If so, then how are they similar and different than one another?arrow_forwardDoes an ordinary electric fan cool the air? Why or why not?If not, why use it?arrow_forward
- For the human body, what is the rate of heat transfer by conduction through the body’s tissue with the following conditions: the tissue thickness is 3.00 cm, the change in temperature is 2.00C, and the skin area is 1.50 m2. How does this compare with the average heat transfer rate to the body resulting from an energy intake of about 2400 kcal per day? (No exercise is included.)arrow_forwardA rectangular window that is 1.20m wide and 1.8m high has a thickness of 6.2 mm and thermal conductivity of 0.2 W/m'C. The temprature inside the home is 21.0 'C and the temperature outside the home is -4.0'C. Calculate the 1)Rate of heat transfer of the window 2) energy transfer by the window after 5.0 minutesarrow_forwardA heater of total radiating surface area of 0.50 m2 is kept at a constant temperature of 100oC. The heater is in a room and keeps the room at a temperature of 25oC. If the emissivity of the heater is 0.8, what is the rate at which the heater supplies heat to the room? σ = 5.67 x 10-8 W/m2K4arrow_forward
- On a hot day, the temperature of an 80,000-L swimming pool increases by 1.50 °C . What is the net heat transfer during this heating? Ignore any complications, such as loss of water by evaporation.arrow_forwardA-2.16. The heat capacity of air is much lower than the heat capacityof water and a relatively small amount of heat is sufficient to change its temperature. Is itone of the reasons why in deserts, despite the high temperature during the day, wit is bitter cold at night. Heat capacity of air in constant volume wroom temperature is approximately 21 J K-1 mol-1. How much heat is needed toIncrease the air temperature in a room of 5.5 mx 6.5 mx 3.0 m by 10 ° C? Howa 1.0 kW heater would have to be on for a long time to achieve this temperature change,if there was no heat lossarrow_forwardA window of a car is found to be covered with 0.9 cm of ice. If the area of the window is 1 m2, and the ice is at -12 degrees Celsius, what is the minimum heat required to melt all the ice? Take the density of ice to be 900 kg/m3, the specific heat capacity of ice to be 0.5 cal/gK and the latent heat of fusion to be 80 cal/g.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Heat Transfer: Crash Course Engineering #14; Author: CrashCourse;https://www.youtube.com/watch?v=YK7G6l_K6sA;License: Standard YouTube License, CC-BY