
Concept explainers
5-114 Carbon dioxide gas, saturated with water vapor, can be produced by the addition of aqueous acid to calcium carbonate based on the following balanced net ionic equation:
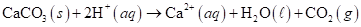
(a) How many moles of wet CO (g), collected at 60.°C and 774 torr total pressure, are produced by the complete reaction of 10.0 g of CaCO3 with excess acid?
(b) What volume does this wet CO2 occupy?
(c) What volume would the CO2 occupy at 774 torr if a desiccant (a chemical drying agent) were added to remove the water? The vapor pressure of water at 60.°C is 149.4 mm Hg.
(a)
Interpretation:
The number of moles of wet
Concept Introduction:
Moles of wet
Answer to Problem 5.114P
The number of moles of carbon dioxide produced is
Explanation of Solution
The weight of calcium carbonate is
The temperature is
The pressure is
The molecular weight of calcium carbonate is calculated below:
The number of moles in
The balanced chemical equation is below:
From the above equation,
Therefore, the number of moles of carbon dioxide produced is
(b)
Interpretation:
The volume of wet
Concept Introduction:
To determine the volume of wet
The relationship between pressure, volume, and temperature, under two sets of conditions, is given by following equation.
Answer to Problem 5.114P
The value of carbon dioxide produced is
Explanation of Solution
The weight of calcium carbonate is
The temperature is.
The pressure is
The molecular weight of calcium carbonate is calculated below:
The number of moles in
The balanced chemical equation is below:
From the above equation:
Therefore, the number of moles of carbon dioxide produced is
We know that at STP, 1 mole of any gas occupies
Therefore,
Therefore, the volume of
At STP, the value of
Now, convert the pressure from
We know that,
Therefore, the pressure is calculated below:
Now, convert temperature from
We know that,
Therefore,
The relationship between pressure, volume, and temperature, under two sets of conditions, is given by following equation:
Upon rearranging, we get,
By substituting the values of
Hence the value of carbon dioxide produced is
(c)
Interpretation:
The volume of wet
Concept Introduction:
To determine the volume of wet
The relationship between pressure, volume, and temperature, under two sets of conditions, is given by following equation.
Answer to Problem 5.114P
Explanation of Solution
The weight of calcium carbonate is
The temperature is
The pressure is
The vapor pressure of water at
The molecular weight of calcium carbonate is calculated below:
The number of moles in
The balanced chemical equation is below:
From the above equation:
Therefore, the number of moles of carbon dioxide produced is
We know that at STP, 1 mole of any gas occupies
Therefore,
Therefore, the volume of
At STP, the value of
The pressure is
Now, convert the pressure from
We know that,
Therefore, the pressure is calculated below,
The vapor pressure of water at
Therefore, the pressure is calculated below,
Now, convert temperature from°C to K.
We know that,
Therefore,
The relationship between pressure, volume, and temperature, under two sets of conditions, is given by following equation,
Upon rearranging, we get,
By substituting the values of
Hence the value of carbon dioxide produced is
Want to see more full solutions like this?
Chapter 5 Solutions
Introduction to General, Organic and Biochemistry
- 5-56 The three main components of dry air and the percentage of each are nitrogen (78.08%), oxygen (20.95%), and argon (0.93%). (a) Calculate the partial pressure of each gas in a sample of dry air at 760 mm Hg. (b) Calculate the total pressure exerted by these three gases combined.arrow_forward5-107 If 60.0 g of NH3 occupies 35.1 L under a pressure of 77.2 in. Hg, what is the temperature of the gas, in °C?arrow_forward5-33 A certain quantity of helium gas is at a temperature of 27 °C and a pressure of 1.00 atm. What will the new temperature be if its volume is doubled at the same time that its pressure is decreased to one-half its original value?arrow_forward
- 5-25 A gas in a bulb as in Figure 5-3 registers a pressure of 833 mm Hg in the manometer in which the reference arm of the U-shaped tube (A) is sealed and evacuated. What will the difference in the mercury levels be if the reference arm of the U-shaped tube is open to atmospheric pressure (760 mm Hg)?arrow_forwardWhen hydrogen peroxide decomposes, oxygen is produced: 2H2O2(aq)2H2O+O2(g)What volume of oxygen gas at 25C and 1.00 atm is produced from the decomposition of 25.00 mL of a 30.0% (by mass) solution of hydrogen peroxide (d=1.05g/mL)?arrow_forward5-111 Diving, particularly SCUBA (Self-Contained Underwater Breathing Apparatus) diving, subjects the body to increased pressure. Each 10. m (approximately 33 ft) of water exerts an additional pressure of 1 atm on the body. (a) What is the pressure on the body at a depth of 100. ft? (b) The partial pressure of nitrogen gas in air at 1 atm is 593 mm Hg. Assuming a SCUBA diver breathes compressed air, what is the partial pressure of nitrogen entering the lungs from a breathing tank at a depth of 100. ft? (c) The partial pressure of oxygen gas in the air at 2 atm is 158 mm Hg. What is the partial pressure of oxygen in the air in the lungs at a depth of 100. ft? (d) Why is it absolutely essential to exhale vigorously in a rapid ascent from a depth of 100. ft?arrow_forward
- 47 HCl(g) reacts with ammonia gas, NH3(g), to form solid ammonium chloride. If a sample of ammonia occupying 250 mL at 21 C and a pressure of 140 torr is allowed to react with excess HCl, what mass of NH4Cl will form?arrow_forward93 The complete combustion of octane can be used as a model for the burning of gasoline: 2C8H18+25O216CO2+18H2O Assuming that this equation provides a reasonable model of the actual combustion process, what volume of air at 1.0 atm and 25°C must be taken into an engine to burn 1 gallon of gasoline? (The partial pressure of oxygen in air is 0.21 atm and the density of liquid octane is 0.70 g/mL.)arrow_forwardGiven that a sample of air is made up of nitrogen, oxygen, and argon in the mole fractions 0.78 N2, 0.21 O2, and 0.010 Ar, what is the density of air at standard temperature and pressure?arrow_forward
- 5-34 A sample of 30.0 mL of krypton gas, Kr, is at 756 mm Hg and 25.0°C. What is the new volume if the pressure is decreased to 325 mm Hg and the temperature is decreased to-12.5°C?arrow_forward5-118 Isooctane, which has a chemical formula C8H18 is the component of gasoline from which the term octane rating derives. (a) Write the balanced chemical equation for the combustion of isooctane. (b) The density of isooctane is 0.792 g/mL. How many kg of C02 are produced each year by the annual U.S. gasoline consumption of L? (c) What is the volume in liters of this CO2 at STP? (d) The chemical formula for isooctane can be represented by (CH3)3CCH2CH(CH3)2. Draw a Lewis structure of isooctane. (e) Another molecule with the same molecular formula is octane, which can be represented by: When comparing isooctane and octane, one structure is observed to have a boiling point of 99°C, while another is known to have a boiling point Of 125°C. Which substance, isooctane or octane, is expected to have the higher boiling point? (f) Determine whether isooctane or octane is expected to have the greater vapor pressure.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





