
Concept explainers
5-118 Isooctane, which has a chemical formula C8H18 is the component of gasoline from which the term octane rating derives.
(a) Write the balanced chemical equation for the combustion of isooctane.
(b) The density of isooctane is 0.792 g/mL. How many kg of C02 are produced each year by the annual U.S. gasoline consumption of 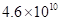 L?
L?
(c) What is the volume in liters of this CO2 at STP?
(d) The chemical formula for isooctane can be represented by (CH3)3CCH2CH(CH3)2. Draw a Lewis structure of isooctane.
(e) Another molecule with the same molecular formula is octane, which can be represented by:
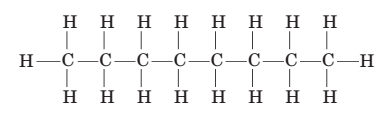
When comparing isooctane and octane, one structure is observed to have a boiling point of 99°C, while another is known to have a boiling point Of 125°C. Which substance, isooctane or octane, is expected to have the higher boiling point?
(f) Determine whether isooctane or octane is expected to have the greater vapor pressure.
(a)
Interpretation:
The balanced chemical equation for the combustion of isooctane should be determined.
Concept Introduction:
In a balanced chemical equation, all the constituents present in the reaction have equal number of atoms on both side of the reaction arrow.
Answer to Problem 5.118P
Explanation of Solution
Isooctane on combustion in the presence of oxygen produces carbon dioxide gas along with water vapor. The balanced chemical equation of the reaction is taking place as depicted below:
(b)
Interpretation:
The mass in kg of CO2 are produced each year by the annual US gasoline consumption should be determined.
Concept Introduction:
Mass of carbon dioxide produces can be obtained from the balanced reaction i.e.
Density of a substance is defined as mass per unit volume. It is mathematically represented as follows:
Here, m is mass and v is volume of the substance.
Answer to Problem 5.118P
Mass of carbon dioxide produces yearly is
Explanation of Solution
Volume of gasoline consumed in 1 year is 4.6 × 1010 L.
Density of the fuel = 0.792 g/mL.
To calculate mass of gasoline, multiply volume of gasoline consumed with its density.
Therefore, the mass of gasoline = 3.64 × 1013 g.
The balanced combustion reaction of gasoline is as follows.
From the balanced reaction,
To calculate the mass of carbon dioxide produces from the
To convert the mass of carbon dioxide from g to kg as follows:
Therefore, mass of carbon dioxide produces yearly is
(c)
Interpretation:
The volume of CO2 in liter at STP should be determined.
Concept Introduction:
Volume CO2 in liter at STP can be determined by calculating moles of CO2 and then using the concept that at STP for each mode of gas occupies 22.4 L.
Answer to Problem 5.118P
The volume of carbon dioxide occupied is
Explanation of Solution
To calculate the number of the moles of carbon dioxide, divide mass of carbon dioxide with its molar mass.
At STP for each mode of gas occupies 22.4 L.
Calculate the volume of carbon dioxide occupied by
Therefore, the volume of carbon dioxide occupied is
(d)
Interpretation:
The Lewis structure of isooctane should be drawn.
Concept Introduction:
Lewis structure are diagrams which represents the bonding between atoms of a molecule and the lone pairs of electrons that might be present in the molecule.
Answer to Problem 5.118P
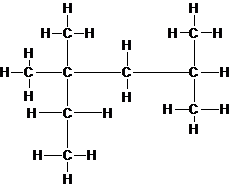
Explanation of Solution
The lewis structure of isooctane is shown in the following diagram. Since, valence electrons in carbon atom are 4 and all the carbon atoms are attached with 4 other atoms (carbon and hydrogen), they have complete octets and all the electrons are engaged in bonding. Thus, there is no lone pair present on any atom.
(e)
Interpretation:
If isooctane or octane is expected to have the higher boiling point should be determined.
Concept Introduction:
One of the parameter for specific boiling point of any liquid can be explained by van der waals bonds.
Liquid having more van der waals bonds will show higher boiling point.
Answer to Problem 5.118P
Isooctance has fewer cohensive interactions than octane so Isooctance has lower boiling point.
Explanation of Solution
Van der Waals attractions increase with the surface areas of the interacting electron clouds. That is, the large the interacting surfaces, the greater the magnitude of the induced dipole. Because isooctance has less surface area at which van der waals interactions with other isooctane molecules can occur, it has fewer cohensive interactions than octane, and thus, a lower boiling point.
(f)
Interpretation:
If isooctane or octane is expected to have the greater vapor pressure should be determined.
Concept Introduction:
Vapor pressure depends on boiling point. lower boiling point shows higher vapor pressure.
Answer to Problem 5.118P
Isooctane is expected to have higher vapour pressure at certain temperature.
Explanation of Solution
As the boiling point of isooctane is lower than Octane, isooctane is expected to have higher vapour pressure at certain temperature.
Want to see more full solutions like this?
Chapter 5 Solutions
Introduction to General, Organic and Biochemistry
- 5-34 A sample of 30.0 mL of krypton gas, Kr, is at 756 mm Hg and 25.0°C. What is the new volume if the pressure is decreased to 325 mm Hg and the temperature is decreased to-12.5°C?arrow_forward5-37 A sample of a gas at 77°C and 1.33 atm occupies a volume of 50.3 L. (a) How many moles of the gas are present? (b) Does your answer depend on knowing what gas it is?arrow_forward5-114 Carbon dioxide gas, saturated with water vapor, can be produced by the addition of aqueous acid to calcium carbonate based on the following balanced net ionic equation: (a) How many moles of wet CO (g), collected at 60.°C and 774 torr total pressure, are produced by the complete reaction of 10.0 g of CaCO3 with excess acid? (b) What volume does this wet CO2 occupy? (c) What volume would the CO2 occupy at 774 torr if a desiccant (a chemical drying agent) were added to remove the water? The vapor pressure of water at 60.°C is 149.4 mm Hg.arrow_forward
- 5-41 Does the density of a gas increase, decrease, or stay the same as the pressure increases at constant temperature? As the temperature increases at constant pressure?arrow_forward5-113 Ammonia and gaseous hydrogen chloride react to form ammonium chloride according to the following equation: If 4.21 L of NH3(g) at 27°C and 1.02 atm is combined with 5.35 L of HCI(g) at 26°C and 0.998 atm, what mass of NH4CI(s) will be generated?arrow_forwardA sample of natural gas is 85.2% methane, CH4, and 14.8% ethane, C2H6, by mass. What is the density of this mixture at 18C and 771 mmHg?arrow_forward
- 109 An ore sample with a mass of 670 kg contains 27.7% magnesium carbonate, MgCO3. If all of the magnesium carbonate in this ore sample is decomposed to form carbon dioxide, describe how to determine what volume of CO2 is evolved during the process. What would have to be measured to predict the needed volume in advance?arrow_forwardA sample of a breathing mixture for divers contained 34.3% helium, He; 51.7% nitrogen, N2; and 14.0% oxygen, O2 (by mass). What is the density of this mixture at 22C and 775 mmHg?arrow_forward5-54 Automobile air bags are inflated by nitrogen gas. When a significant collision occurs, an electronic sensor triggers the decomposition of sodium azide to form nitrogen gas and sodium metal. The nitrogen gas then inflates nylon bags, which protect the driver and front-seat passenger from impact with the dashboard and windshield. What volume of nitrogen gas measured at 1 atm and 27°C is formed by the decomposition of 100. g of sodium azide?arrow_forward
- 5-33 A certain quantity of helium gas is at a temperature of 27 °C and a pressure of 1.00 atm. What will the new temperature be if its volume is doubled at the same time that its pressure is decreased to one-half its original value?arrow_forward5-46 Calculate the molar mass of a gas if 3.30 g of the gas occupies 660. mL. at 735 mm Hg and 27°C.arrow_forwardUranium hexafluoride, UF6, is a white solid that sublimes (vaporizes without melting) at 57C under normal atmospheric pressure. The compound is used to separate uranium isotopes by effusion. What is the rms speed (in m/s) of a uranium hexafluoride molecule at 57C?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





