
Concept explainers
(a)
Interpretation:The isomeric relationship between cyclopropylcyclopentane and cyclobutylcyclobutane should beidentified.
Concept introduction:Chiral carbon is any stereocenter attached to four different alkyl substituents. If any two of the substituent happen to be similar the center is regarded as achiral.
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold similar mirror image relationships.
The molecular models of

The two compounds constitute a pair of constitutional isomers. These kinds of isomers are formed by cleavage and replacement of bonds, unlike the conformational isomers that result due to rotation around the bond.
(a)
Explanation of Solution
Structures of cyclopropylcyclopentane and cyclobutylcyclobutane are illustrated as follows:

The molecular formula for both cyclopropylcyclopentane and cyclobutylcyclobutane is
(b)
Interpretation: The isomeric relationship among
Concept introduction: Chiral carbon is any stereocenter attached to four different alkyl substituents. If any two of the substituent happen to be similar the center is regarded as achiral.
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold similar mirror image relationships.
The molecular models of

The two compounds constitute a pair of constitutional isomers. These kinds of isomers are formed by cleavage and replacement of bonds, unlike the conformational isomers that result due to rotation around the bond.
(b)
Explanation of Solution
Structures of
are illustrated as follows:
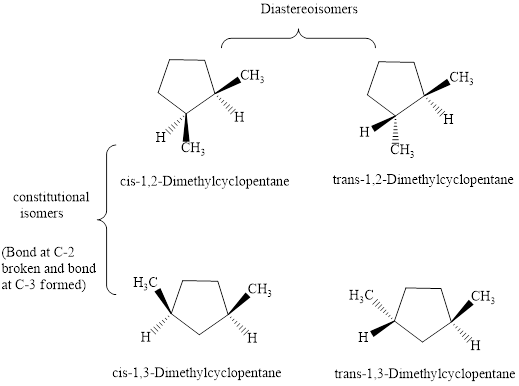
Since cis and trans form represent diastereoisomers therefore
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry: Structure and Function
- Consider 1-bromo-2-methylpropane and draw the following. (a) The staggered conformation(s) of lowest energy (b) The staggered conformation(s) of highest energyarrow_forwardThe heat of hydrogenation of cis-2,2,5,5-tetramethyl-3-hexene is -154 kJ (-36.7 kcal)/ mol, while that of the trans isomer is only -113 kJ (-26.9 kcal)/mol. Q.) If a catalyst could be found that allowed equilibration of the cis and trans isomers at room temperature (such catalysts do exist), what would be the ratio of trans to cis isomers?arrow_forwardKnowing that cyclohexane and hexene are isomers, suggest 2 methods allowing their separation.arrow_forward
- Consider 1-bromopropane, CH3CH2CH2Br. (a) Which of these is the lowest energy conformation?arrow_forwardUsing what you know about the conformational energetics of substituted cyclohexanes, predict which of the two decalin isomers is more stable. Explain your reasoning.arrow_forwardTwo alkenes undergo hydrogenation to yield a mixture or cis- and trans-1,4-dimethylcyclohexane. Which two are these? A third, however, gives only cis-1,4-dimethylcyclohexane. What compound is this?arrow_forward
- What is the most stable conformation for trans-1,2-dichlorocyclohexane. Describe and explain brieflyarrow_forwardRead Appendix B on naming branched alkyl substituents, and draw all possible alkyl groups having the formula C5H11–. Give the IUPAC names for the eight compounds of molecular formula C10H20 that contain a cyclopentane ring with each of these alkyl groups as a substituent.arrow_forwardWhy are these two conformational isomers? I was under the impression that in molecules with only sigma bonds, the bonds can rotate freely, and so the placement of the chlorines wouldn't matter?arrow_forward
- For the 1,2-dichlorocyclohexane stereoisomers, which conformation of the trans stereoisomer has the lower energy, the diaxial or the diequatorial conformer? Which isomer and conformation of the three you built has the lowest energy? E of all the isomers of 1,2-dichlorocyclohexane. Explain the differences in energy, i.e., identify the sources of strain in the conformations you built. Show the sources of strain in a drawing.arrow_forwardbenzene, C6H6, gives thecompound special stability.(a) By using data in Appendix C, compare the heat of combustionof 1.0 mol C6H6(g) to the heat of combustion of 3.0 mol acetylene,C2H2(g). Which has the greater fuel value, 1.0 mol C6H6(g) or 3.0mol C2H2(g)? Are your calculations consistent with benzene beingespecially stable?arrow_forwardDraw the structure(s) of all of the alkene isomers, C5H10, that contain a branched chain. Consider E/Z stereochemistry of alkenes.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
