
Concept explainers
(a)
Interpretation:
The amount of pressure created when a gas molecule moving in a closed container has to be explained.
Concept Introduction:
Gas pressure
Pressure or Stress is the force applied perpendicular to the surface of an object per unit area.
SI derived unit of pressure is Pascal (Pa).
(a)
Answer to Problem 5.28QP
The pressure in the closed container developed when the gas molecule has a constant bombardment or collisions with the sides of the containers.
Explanation of Solution

Figure 1
According to kinetic theory of gases the molecules in a container are continuously moving freely. The molecule undergoes collisions within themselves and with the sides of the container which are very small but on an average the collisions are considerable forces on the container. The molecule exerts maximum force at the surface if the angle of incidence is 900.
The summation of all the forces are known as pressure.
The amount of pressure created when a gas molecule moving in a closed container is explained.
(b)
Interpretation:
The amount of pressure created when the gas molecule(s) moving in closed containers A and B has to be explained.
Concept Introduction:
Gas pressure:
Pressure or Stress is the force applied perpendicular to the surface of an object per unit area.
SI derived unit of pressure is Pascal (Pa).
(b)
Answer to Problem 5.28QP
Container B exerts more pressure than the Container A.
Explanation of Solution
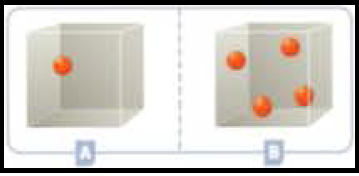
Figure 2
The pressure in the closed container developed when the gas molecules has a constant bombardment or collisions with the sides of the containers.
According to
Since container B having more number of molecules than the container A, container B exerts more pressure.
The amount of pressure created when different number of gas molecules in a closed containers is explained.
(c)
Interpretation:
The amount of pressure has to be explained when the containers C and D contains atoms and molecules.
Concept Introduction:
Gas pressure:
Pressure or Stress is the force applied perpendicular to the surface of an object per unit area.
SI derived unit of pressure is Pascal (Pa).
(c)
Answer to Problem 5.28QP
The pressure in container B is four times the pressure in container A. Since Container B contains four times the molecules in container A.
Explanation of Solution
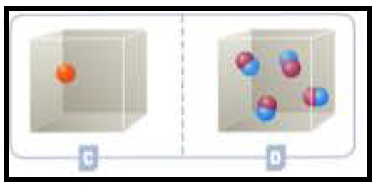
Figure 3
According to Kinetic theory of gases, the more the number of molecules, the more pressure will be in the container. Since container B having four molecules whereas container A has one atom, container B having more pressure than the container A. container is independent of mass but dependent on no of particles.
The amount of pressure was explained when the containers contains atom and molecules.
(d)
Interpretation:
Among the given containers C and D, the container with highest RMS value has to be identified and answer has to be contradicted with the pressure of the container.
Concept Introduction:
Root mean square value:
Where,
(d)
Answer to Problem 5.28QP
Since the atom is lighter than the molecules it will have higher rms speed.
Explanation of Solution
The RMS speed is directly proportional to the square root of
RMS speed doesn’t depends on the pressure of the container, so the pressure of the neither gets support nor contradicts with the RMS value. Because force on the sides of the container doesn’t depend on speed of the particle alone, it does depend on the momentum of the particle
Finally the RMS speed depends on temperature. At constant temperature constant force will be there.
Among the given containers C and D, the container with highest RMS value has been identified and answer has contradicted with the pressure of the container.
(e)
Interpretation:
At the given conditions the pressures among the Containers E and F should be compared.
Concept Introduction:
Gay-Lussac’s law:
Gay-Lussac law derived from the Charles law,
The law states that a sample gas having fixed pressure is directly proportional to the temperature. Since pressure can never be negative and the temperature scale should be absolute minimum.
So the initial and final Pressure will be like
Here,
P1 and P2 are initial and final Pressure.
T1 and T2 are initial and final Temperature.
(e)
Answer to Problem 5.28QP
The pressure in the container F doubles than the pressure in the Container E.
Explanation of Solution
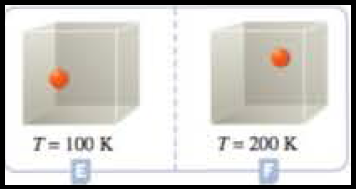
Figure 4
According to Gay lussac law, at equal volumes and equal number of molecules pressure and temperature are directly proportional to each other. So, the containers E and F has equal volumes and equal number of molecules. The temperature in the container F is T = 200 K, and Container E has T = 100 K, according to Gay-Lussac law container F contains double the pressure then the container E.
At the given conditions the pressures among the Containers E and F was compared.
(f)
Interpretation:
At the given conditions the pressures among the Containers G and H should be compared.
Concept Introduction:
Gay-Lussac’s law:
Gay-Lussac law derived from the Charles law,
The law states that a sample gas having fixed pressure is directly proportional to the temperature. Since pressure can never be negative and the temperature scale should be absolute minimum.
So the initial and final Pressure will be like
Here,
P1 and P2 are initial and final Pressure.
T1 and T2 are initial and final Temperature.
(f)
Answer to Problem 5.28QP
The pressure in the container H doubles than the pressure in the Container G.
Explanation of Solution
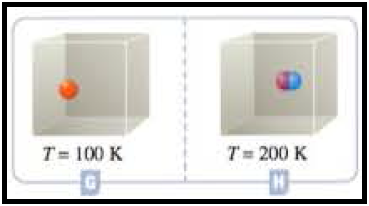
Figure 5
According to Gay lussac law, at equal volumes and equal number of molecules pressure and temperature are directly proportional to each other. So, the containers H and G has equal volumes and equal number of molecules. The temperature in the container H is T = 200 K, and Container G has T = 100 K, according to Gay-Lussac law container H contains double the pressure then the container G. pressure of the container doesn’t depends on the mass of the particle.
At the given conditions the pressures among the Containers G and H was compared.
(g)
Interpretation:
At the given conditions the pressures among the Containers G and H should be compared.
Concept Introduction:
Gay-Lussac’s law:
Gay-Lussac law derived from the Charles law,
The law states that a sample gas having fixed pressure is directly proportional to the temperature. Since pressure can never be negative and the temperature scale should be absolute minimum.
So the initial and final Pressure will be like
Here,
P1 and P2 are initial and final Pressure.
T1 and T2 are initial and final Temperature.
(g)
Answer to Problem 5.28QP
Both the containers I and J will have same pressures.
Explanation of Solution
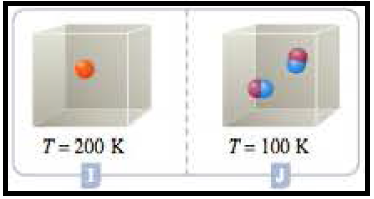
Figure 6
According to Gay-Lussac law, pressure of the container relates to Temperature and number of molecules as well. Here the temperature in container I doubles the temperature J which adequately doubles the pressure. But the number of particles in container J is the double the number of particles in container I, which effectively reduces the pressure by one-half. As whole the pressures in the containers remain same.
At the given conditions the pressures among the Containers G and H was compared.
(h)
Interpretation:
At the given conditions the pressures among the Containers K and L should be compared.
Concept Introduction:
Gay-Lussac’s law:
Gay-Lussac law derived from the Charles law,
The law states that a sample gas having fixed pressure is directly proportional to the temperature. Since pressure can never be negative and the temperature scale should be absolute minimum.
So the initial and final Pressure will be like
Here,
P1 and P2 are initial and final Pressure.
T1 and T2 are initial and final Temperature.
(h)
Answer to Problem 5.28QP
The pressure in the container K six times than the pressure in the Container L
Explanation of Solution
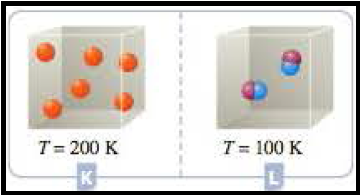
Figure 7
Here in this case, container K has six atoms and T = 200 K. whereas container L has 2 molecules and T=100 K. So the Container K has three times than the particles in container L and the temperature doubles than container L. So the container K has six times more pressure than the container L.
At the given conditions the pressures among the Containers K and L was compared.
Want to see more full solutions like this?
Chapter 5 Solutions
General Chemistry - Standalone book (MindTap Course List)
- Shown below are three containers of an ideal gas (A, B, and C), each equipped with a movable piston (assume that atmospheric pressure is 1.0 atm). a How do the pressures in these containers compare? b Are all the gases at the same temperature? If not, compare the temperatures. c If you cooled each of the containers in an ice-water bath to 0.0C, describe how the volumes and pressures of the gases in these containers would compare.arrow_forwardThe graph below shows the distribution of molecular speeds for helium and carbon dioxide at the same temperature. (a) Which curve could represent the behavior of carbon dioxide? (b) Which curve represents the gas that would effuse more quickly? (c) Which curve could represent the behavior of helium gas?arrow_forwardA collapsed balloon is filled with He to a volume of 12.5 L at a pressure of 1.00 atm. Oxygen, O2, is then added so that the final volume of the balloon is 26 L with a total pressure of 1.00 atm. The temperature, which remains constant throughout, is 21.5 C. (a) What mass of He does the balloon contain? (b) What is the final partial pressure of He in the balloon? (c) What is the partial pressure of O2 in the balloon? (d) What is the mole fraction of each gas?arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





