
Concept explainers
(a)
Interpretation: The structure of naturally occurring
Concept introduction: A compound exhibits number of stereoisomers when it contains more than one stereogenic centers. The maximum number of stereoisomers a compound with
Answer to Problem 5.55P
The structure of naturally occurring
Explanation of Solution
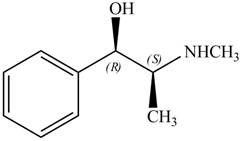
The structure of given compoundis shown below.
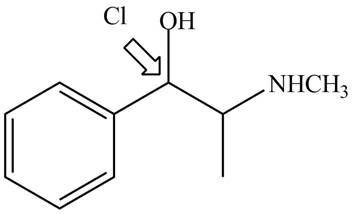
Figure 1
The stereochemistry of the compound is determined by prioritizing the groups attached to its stereogenic center. The groups are prioritizedon the basis ofatomic number of their atoms. The group that contains atom with a the higher
The configuration at the first stereocenter of
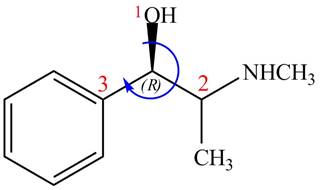
Figure 2
The above structure implies that the configuration at the first stereocenter of
The configuration at the second stereocenter of
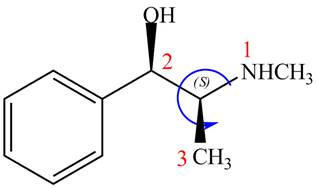
Figure 3
The above structure implies that the configuration at the second stereocenter of
The configuration at both the stereocenters of
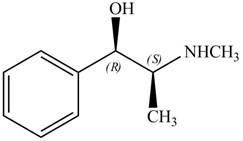
Figure 4
The configurations at the first and second stereocenters of
The structure of naturally occurring
(b)
Interpretation: The structure of naturally occurring
Concept introduction: A compound exhibits number of stereoisomers when it contains more than one stereogenic centers. The maximum number of stereoisomers a compound with
Answer to Problem 5.55P
The structure of naturally occurring
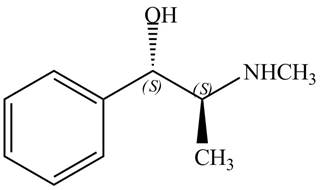
Explanation of Solution
The structure of naturally occurring
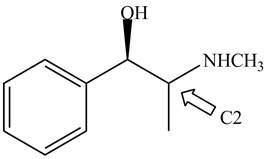
Figure 5
The stereochemistry of the compound is determined by prioritizing the groups attached to its stereogenic center. The groups are prioritizedon the basis of atomic number of their atoms. The group that contain atom with higher atomic number is givenhigher priority. Complete the circle in decreasing order of priorityfrom
The configuration at first chiral center of
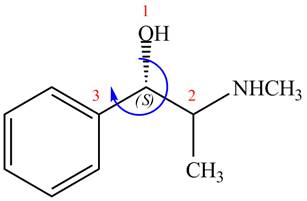
Figure 6
The above structure implies that the configuration at the first stereocenter of
The configuration at the second chiral center of
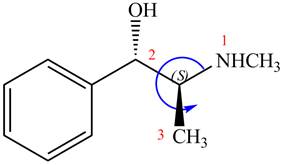
Figure 7
The above structure implies that the configuration at the second stereocenter of
The configuration at the first and second chiral centers of
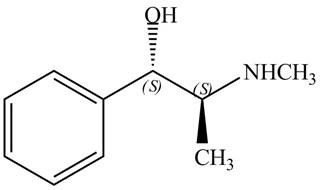
Figure 8
The configurations at the first and second carbon atoms of
The structure of naturally occurring
(c)
Interpretation: The relationship between ephedrine and pseudoephedrine is to be identified.
Concept introduction: A compound exhibits number of stereoisomers when it contains more than one stereogenic centers. The maximum number of stereoisomers a compound with
Answer to Problem 5.55P
Ephedrine and pseudoephedrine are related as diastereomers.
Explanation of Solution
The relationship between ephedrine and pseudoephedrine is shown below.
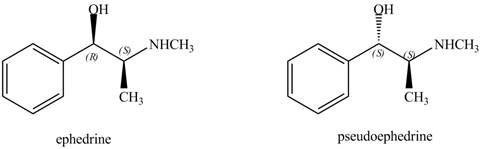
Figure 9
One stereocenter in both the compounds has opposite configuration, while the other stereogenic center has the same configuration. Therefore, both the compounds are related as diastereomer.
Ephedrine and pseudoephedrine are related as diastereomers.
(d)
Interpretation: All the stereoisomers of
Concept introduction: A compound exhibits number of stereoisomers when it contains more than one stereogenic centers. The maximum number of stereoisomers a compound with
Answer to Problem 5.55P
The stereoisomers of
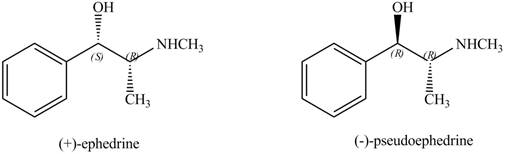
Explanation of Solution
The stereoisomers of
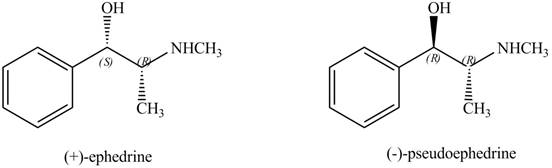
Figure 10
Both the stereoisomers have two stereocenters. One stereocenter in both the compounds has the same configuration, while the other stereocenter in both the compounds has opposite configuration.
The stereoisomers of
(e)
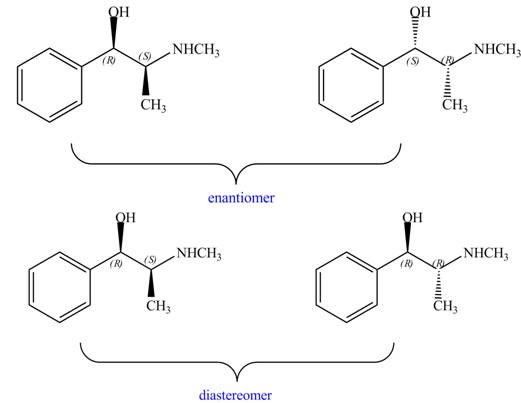
Interpretation: The relationship between each compound drawn in part (d) and
Concept introduction: A compound exhibits number of stereoisomers when it contains more than one stereogenic centers. The maximum number of stereoisomers a compound with
Answer to Problem 5.55P
The relationship between each compound drawn in part (d) and
Explanation of Solution
The relationship between each compound drawn in part (d) and

Figure 11
The compounds
The relationship between each compound drawn in part (d) and
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry
- A chemist isolated an aromatic compound with molecular formula C6H4Br2. He treated this compound with nitric acid and sulfuric acid and isolated threedifferent isomers, in different amounts, with molecular formula C6H3Br2NO2. What was the structure of the original compound?arrow_forwardA chiral alkyne A with molecular formula C6H10 is reduced with H2 and Lindlar catalyst to B having the R conguration at its stereogenic center. What are the structures of A and B?arrow_forwardPlants extract are widely used in many parts of Cameroon to treat infectious disease or related symptoms including abdominal pains, itching, urinary and respiratory ailments, fever, coughing and diarrhea. Harunmadagascarin C as depicted below is an extract from the plant Harungana madagascariensis that has been studied for potential antimicrobial activity. Which statement best describes the stereochemistry of the compound? a. Harunmadagascarin C is achiral and does not have any stereogenic or chiral centers. b. The two stereogenic centers are currently in the E configuration, and the double bond in the ring structure is not stereogenic. c. There are four possible stereoisomers for Harunmadagascarin C since, as depicted, there are 2 stereocenters in the compound. d. There is one chiral center present in the molecule.arrow_forward
- DHA is a fatty acid derived from sh oil and an abundant fatty acid in vertebrate brains. Hydrogenation of DHA forms docosanoic acid [CH3(CH2)20CO2H] and ozonolysis forms CH3CH2CHO, CH2(CHO)2 (ve equivalents), and HCOCH2CH2CO2H. What is the structure of DHA if all double bonds have the Z conguration?arrow_forward4-Chloro-2-pentene has a double bond that can have either the E or the Z configuration and a stereogenic center that can have either the R or the S configuration. How many stereoisomers arepossible altogether? Draw the structure of each, and group the pairs of enantiomers.arrow_forwardThe compound MON-0585 is a non-toxic, biodegradable larvicide that is highly selective against mosquito larvae. Synthesize MON-0585 using either benzene or phenol as a source of the aromatic rings.arrow_forward
- Compounds A and B are isomers having molecular formula C5H12. Heating A with Cl2 gives a single product of monohalogenation, whereas heating B under the same conditions forms three constitutional isomers. What are thestructures of A and B?arrow_forwardAn unknown compound A of molecular formula C10H18O reacts with H2SO4 to form two compounds (B and C)of molecular formula C10H16. B and C both react with H2 in the presence of Pd-C to form decalin. Ozonolysis of B forms D, and ozonolysis of C forms a diketone E of molecular formula C10H16O2. Identify the structures of compounds A, B, C, and E.arrow_forwardDraw the structure of (S,S)-ethambutol, a drug used to treat tuberculosis that is 10 times more potent than any of its other stereoisomers.arrow_forward
- Compound A is an aromatic compound with the molecular formula C8H8. When treated with excess BR2, compound A is converted into compound B, with the molecular formula C8H8BR2. Draw the structure of compound a. arrow_forwardFollowing is the structure formula for Sertaline(Zoloft), widely prescribed to treat depression. Circle all the sterocenters present- how many stereoisomers of Zoloft are possible? Of these stereoisomers, how many are likely to be biologically effective in treating depression? Explain briefly.arrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
