
Concept explainers
Predict all approximate bond angles about each highlighted carbon atom. To make these predictions, use valence-shell electron-pair repulsion (VSEPR) theory (Section 1.4).
(a) 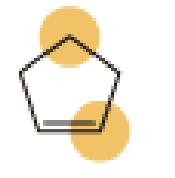
(b) 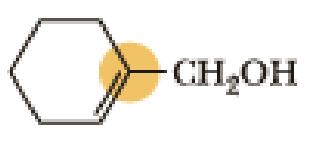
(c) 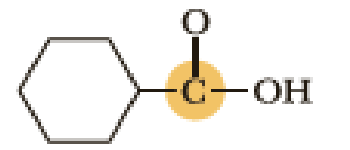
(d) 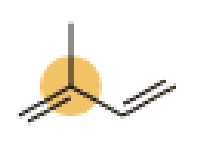
(e) 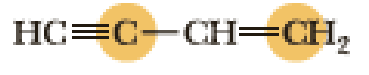
(a)
Interpretation:
An approximate bond angle of the highlighted carbon atom has to be predicted using VSEPR theory.
Concept Introduction:
VSEPR Theory:
The basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions.
According to VSEPR theory, the geometry is predicted by the minimizing the repulsions between electron-pairs in the bonds and lone-pairs of electrons. The VSEPR theory is summarized in the given table as,
- • Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
- • Electro-domain geometry includes both bond pairs and lone pairs of central atom for determining the geometry of molecule.
Explanation of Solution
Bond angle can be determined based on the electron-domain geometry.
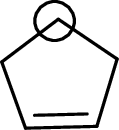
The marked carbon atom has four bond pairs. Thus it has four electron domains and is in tetrahedral geometry. Hence, the bond angle is
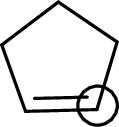
The marked carbon atom has three bond pairs. Thus it has three electron domains and is in trigonal planar geometry. Hence, the bond angle is
(b)
Interpretation:
An approximate bond angle of the highlighted carbon atom has to be predicted using VSEPR theory.
Concept Introduction:
VSEPR Theory:
The basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions.
According to VSEPR theory, the geometry is predicted by the minimizing the repulsions between electron-pairs in the bonds and lone-pairs of electrons. The VSEPR theory is summarized in the given table as,
- • Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
- • Electro-domain geometry includes both bond pairs and lone pairs of central atom for determining the geometry of molecule.
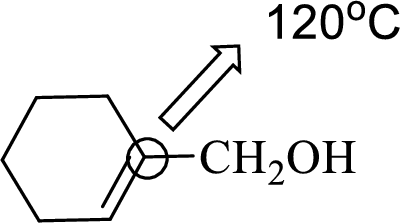
Explanation of Solution
Bond angle can be determined based on the electron-domain geometry.
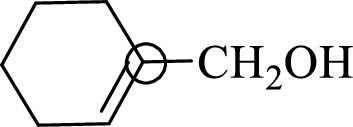
The marked carbon atom has three bond pairs. Thus it has three electron domains and is in trigonal planar geometry. Hence, the bond angle is
(c)
Interpretation:
An approximate bond angle of the highlighted carbon atom has to be predicted using VSEPR theory.
Concept Introduction:
VSEPR Theory:
The basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions.
According to VSEPR theory, the geometry is predicted by the minimizing the repulsions between electron-pairs in the bonds and lone-pairs of electrons. The VSEPR theory is summarized in the given table as,
- • Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
- • Electro-domain geometry includes both bond pairs and lone pairs of central atom for determining the geometry of molecule.
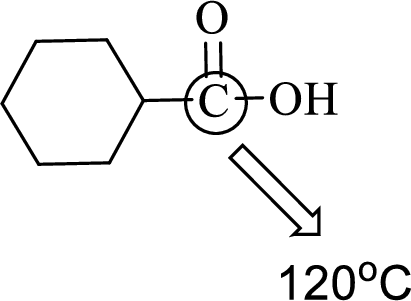
Explanation of Solution
Bond angle can be determined based on the electron-domain geometry.
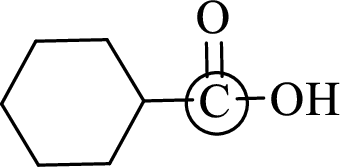
The marked carbon atom has three bond pairs. Thus it has three electron domains and is in trigonal planar geometry. Hence, the bond angle is
(d)
Interpretation:
An approximate bond angle of the highlighted carbon atom has to be predicted using VSEPR theory.
Concept Introduction:
VSEPR Theory:
The basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions.
According to VSEPR theory, the geometry is predicted by the minimizing the repulsions between electron-pairs in the bonds and lone-pairs of electrons. The VSEPR theory is summarized in the given table as,
- • Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
- • Electro-domain geometry includes both bond pairs and lone pairs of central atom for determining the geometry of molecule.
Explanation of Solution
Bond angle can be determined based on the electron-domain geometry.
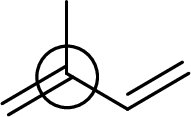
The marked carbon atom has three bond pairs. Thus it has three electron domains and is in trigonal planar geometry. Hence, the bond angle is
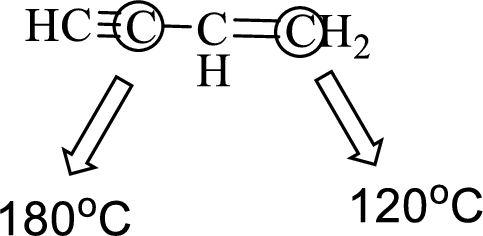
(e)
Interpretation:
An approximate bond angle of the highlighted carbon atom has to be predicted using VSEPR theory.
Concept Introduction:
VSEPR Theory:
The basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions.
According to VSEPR theory, the geometry is predicted by the minimizing the repulsions between electron-pairs in the bonds and lone-pairs of electrons. The VSEPR theory is summarized in the given table as,
- • Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
- • Electro-domain geometry includes both bond pairs and lone pairs of central atom for determining the geometry of molecule.
Explanation of Solution
Bond angle can be determined based on the electron-domain geometry.
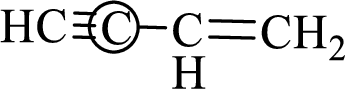
The marked carbon atom has two bond pairs. Thus it has two electron domains and is in linear geometry. Hence, the bond angle is
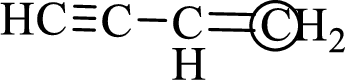
The marked carbon atom has three bond pairs. Thus it has three electron domains and is in trigonal planar geometry. Hence, the bond angle is
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry
- Write each of the following condensed structural formulas as a bond-line formula (that is, using only lines and no elemental symbols). (a) CH3CH2CH2CH2CH3 (b) (CH3)2CHCH2CH3arrow_forwardBenzene: Give an explanation for why there is no directionality for a substituent group coming off of benzene. Savedarrow_forwardDraw the structure of all compounds that fit the following descriptions. a. five constitutional isomers having the molecular formula C4H8 b. nine constitutional isomers having the molecular formula C7H16 c. twelve constitutional isomers having the molecular formula C6H12 and containing one ringarrow_forward
- Draw the structure of all compounds that fit the following descriptions. a.five constitutional isomers having the molecular formula C4H8 b.nine constitutional isomers having the molecular formula C7H16 c.twelve constitutional isomers having the molecular formula C6H12 and containing one ringarrow_forwardIn each of the following classes of organic compounds, write a molecule with a total of 16 carbons (by express formula) with at least one aromatic group and one alkyl group in its structure. Name each structure you write according to the IUPAC system. a) Write and name an aromatic dinitro molecule.b) Write and name a heteroaromatic molecule.c) Write and name a benzaldehyde derivative.arrow_forwardRank the three compounds below in order of shortest to longest carbon–oxygen bond length and explain your reasoning A)Tetrahydro-2H-pyran-2-one B)cyclohexane C)1-Methyl-2-piperidinonearrow_forward
- Write a molecule (with its open formula), which is aromatic for each of the following items, with a total of 14 carbons, together with the side groups. Name each structure you write according to the IUPAC system. a)Write and name an aromatic dinitrile molecule. b)Write and name a heteroaromatic molecule. c)Write and name the aromatic molecule containing an alkenyl group in a side group.arrow_forwardS.1. Write a molecule with a total of 16 carbons (by express formula) in each of the following classes of organic compounds, with at least one aromatic group and one alkyl group in their structure. Name each structure you write according to the IUPAC system. a) Write and name a fused ring aromatic molecule. b) Write down and name the molecule like a meta-disubstitute. c) Write down and name an alkynylphenol molecule.arrow_forwardTaxol is an anti-cancer chemotherapy drug. It is used for the treatment of solid tumors cancers like lung, breast, and ovarian cancer to name a few. Identify the correct functional group that is encircled on Taxol’s chemical structure.arrow_forward
- Provide the two isomers of compound A. Please provide the IUPAC name for each. 1st isomer: has quarternary, secondary, and primary carbons 2nd isomer: has secondary and primary carbonsarrow_forwardIn each of the following classes of organic compounds, write a molecule with a total of 16 carbons (by express formula) with at least one aromatic group and one alkyl group in its structure. Name each structure you write according to the IUPAC system. a) Write and name a fused ring aromatic molecule.b) Write down and name the molecule like a meta-disubstitute.c) Write down and name an alkynylphenol molecule.arrow_forwardGive the IUPAC name of all three subparts in clear handwritten paper!arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
