
A tank with an internal volume of 1 m3 contains air at 800 kPa and 25°C. A valve on the tank is opened, allowing air to escape, and the pressure inside quickly drops to 150 kPa, at which point the valve is closed. Assume there is negligible heat transfer from the tank to the air left in the tank.
- (a) Using the approximation he ≈ constant = he,avg = 0.5 (h1 + h2), calculate the mass withdrawn during the process.
- (b) Consider the same process but broken into two parts. That is, consider an intermediate state at P2 = 400 kPa, calculate the mass removed during the process from P1 = 800 kPa to P2 and then the mass removed during the process from P2 to P3 = 150 kPa, using the type of approximation used in part (a), and add the two to get the total mass removed.
- (c) Calculate the mass removed if the variation of he is accounted for.
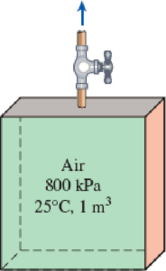
FIGURE P5–185
(a)
The mass withdrawn during the process.
Answer to Problem 185RP
The mass withdrawn during the process is
Explanation of Solution
Write the equation of mass balance.
Here, the inlet mass is
The change in mass of the system for the control volume is expressed as,
Here, the suffixes 1 and 2 indicates the initial and final states of the system.
Consider the tank as the control volume. Initially the tank is filled with air and the valve is in closed position, further no other mass is allowed to enter the tank. Hence, the inlet mass is neglected i.e.
Rewrite the Equation (I) as follows.
Write the formula for initial and final mass of air present in the tank.
Here, the mass of air is
Write the energy balance equation.
Here, the heat transfer is
When the valve is opened and air starts escape from the tank. Neglect the heat transfer and work done i.e.
The Equation (V) reduced as follows.
The enthalpy and internal energy in terms of temperature and specific heats are expressed as follows.
Rewrite the Equation (VI) as follows.
The temperature of the air while exiting the tank is considered as the average temperature of initial and final temperatures.
Refer Table A-1, “Molar mass, gas constant, and critical-point properties”.
The gas constant
Refer Table A-2b, “Ideal-gas specific heats of various common gases”.
The specific heat at constant pressure
Conclusion:
Substitute
Substitute
Substitute
Use Engineering Equation Solver (EES) or online calculator to solve the Equation (VIII) and obtain the value of
Substitute
Substitute
Thus, the mass withdrawn during the process is
(b)
The mass withdrawn during the pressure reduced from
Answer to Problem 185RP
The total mass withdrawn during the process 1-3 is
Explanation of Solution
Consider Process 1-2:
The pressure drop from
Substitute
Substitute
Substitute
Use Engineering Equation Solver (EES) or online calculator to solve the Equation (IX) and obtain the value of
Substitute
Substitute
Thus, the mass withdrawn during the process 1-2 is
Consider Process 2-3:
The pressure drop from
Here,
Substitute
Substitute
Substitute
Use Engineering Equation Solver (EES) or online calculator to solve the Equation (X) and obtain the value of
Substitute
Substitute
Thus, the mass withdrawn during the process 2-3 is
The total mass withdrawn during the process 1-3 is as follows.
Thus, the total mass withdrawn during the process 1-3 is
(c)
The mass withdrawn during the process if there is variation in
Answer to Problem 185RP
The mass withdrawn during the process is
Explanation of Solution
Write the general mass balance equation.
Here, the inlet mass flow rate is
Refer Equation (XI).
Write the mass balance equation for the given system.
Rewrite the Equation (XII) as follows.
Write the general energy rate balance equation.
Here, the rate of total energy in is
The system is at steady state. Hence, the rate of change in net energy of the system becomes zero.
Refer Equation (XIII).
Write the energy balance equation for the given system.
Here, the mass is
Substitute
The enthalpy and internal energy is expressed as follows.
Substitute
The mass of air in terms ideal gas is expressed as follows.
Rewrite the Equation (XVI) as follows.
Using
Substitute
Here,
Integrate the Equation (XVIII) at the initial-1 and final-2 states.
Refer Table A-2(a), “Ideal-gas specific heats of various common gases”.
The specific heat ratio
Conclusion:
Substitute
Substitute
Substitute
Thus, the mass withdrawn during the process is
Want to see more full solutions like this?
Chapter 5 Solutions
Thermodynamics: An Engineering Approach
Additional Engineering Textbook Solutions
Mechanics of Materials (10th Edition)
Degarmo's Materials And Processes In Manufacturing
Automotive Technology: Principles, Diagnosis, And Service (6th Edition) (halderman Automotive Series)
Fundamentals of Heat and Mass Transfer
Automotive Technology: Principles, Diagnosis, and Service (5th Edition)
Shigley's Mechanical Engineering Design (McGraw-Hill Series in Mechanical Engineering)
- Water vapor enters a turbine at 6 MPa and 400C, and leaves the turbine at 100 kPa with the same specific entropy as that at the inlet. Calculate the difference between the specific enthalpy of the water at the turbine inlet and exit.arrow_forwardWater is contained in a closed rigid tank at an initial pressure of 1200 kPa. Heat transfer occurs until the pressure increases to 7 MPa and the tank found to be containing 1,78 kg saturated liquid, and the mass of the saturated vapor is 0.22 kg. Determine the initial temperature, entropy and enthalpy of the water.arrow_forward10 kg/s of air enters the turbine at a pressure of 120 MPa and 910 K. The air expands Isentropically to a pressure of 25 MPa, determine the Hp of this turbine. Also draw and label the P-V and T-S diagram.arrow_forward
- In an open system, 500 kg/h of air is being compressed from 100 kPa at 280 K to 600 kPa and 400 K. The air enters the compressor at a linear velocity of 50 m/s and leaves at a velocity of 100 m/s. The power required for the compressor is 50 kW, and the heat loss from the turbine is estimated to be 10,000 kJ/h. Determine the specific enthalpy change that associated with the process. The process is in steady state and has no change in its mass flow. (1 kW = 3600 kJ/h)arrow_forwardSteam flows steadily through an adiabatic turbine. The inlet conditions of the steam are 4 MPa, 500°C, and 80 m/s, and the exit conditions are 30 kPa, 92 percent quality, and 50 m/s.arrow_forwardAir at an initial state of 100 kpa and 17 degree Celsius is compressed to a final state of 600 kpa and 57 degree Celsius. Sketch the T-s diagram and determine the entropy changes of this process using property values from air tables for exact analysis.arrow_forward
- Which of the two gases—helium or nitrogen—has the higher final temperature as it is compressed isentropically from 100 kPa and 25°C to 1 MPa in a closed system?arrow_forwardAir is compressed by an adiabatic compressor from 95 kPa and 27°C to 600 kPa and 277°C. Assuming variable specific heats and neglecting the changes in kinetic and potential energies, determine the exit temperature of air if the process were reversible.arrow_forwardTwo rigid tanks are connected by a valve. Tank A contains 0.6 m^3 of water at 330 kPa and 90 percent quality. Tank B contains 0.5 m^3 of water at 250 kPa and 250 ∘C. The valve is then opened, and the two tanks eventually come to equilibrium while exchanging heat with the surroundings at 25 ∘C. Determine the final pressure, heat transfer, and entropy generation.arrow_forward
- Three kg of helium gas at 100 kPa and 27°C are adiabatically compressed to 900 kPa. If the isentropic compression efficiency is 80 percent, determine the required work input and the final temperature of helium.arrow_forwardA 0.8-m3 rigid tank contains carbon dioxide (CO2) gas at 250 K and 100 kPa. A 500-W electric resistance heater placed in the tank is now turned on and kept on for 40 min, after which the pressure of CO2 is measured to be 175 kPa. Assuming the surroundings to be at 300 K and using constant specific heats, determine the entropy generation during this process.arrow_forwardSteam enters an adiabatic turbine steadily at 7 MPa, 500°C, and 45 m/s and leaves at 100 kPa and 75 m/s. If the power output of the turbine is 5 MW and the isentropic efficiency is 77 percent, determine the mass flow rate of steam through the turbine.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





