
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.9, Problem 12P
Carbocations are key intermediates in petroleum refining. Of particular importance is one having the carbon skeleton shown.
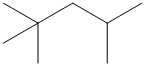
How many different
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
On a cyclohexane ring, an axial carboxyl group has a conformational energy of 5.9 kJ (1.4 kcal)/mol relative to an equatorial carboxyl group. Consider the equilibrium for the alternative chair conformations of trans-1,4-cyclohexanedicarboxylic acid. Draw the less stable chair conformation on the left of the equilibrium arrows and the more stable chair on the right. Calculate DG0 for the equilibrium as written and calculate the ratio of the more stable chair to the less stable chair at 25°C.
3,4-Dimethylpent-1-ene has the formula CH2“CH¬CH(CH3)¬CH(CH3)2. When pure (R)-3,4-dimethylpent-1-ene is treated with hydrogen over a platinum catalyst, the product is (S)-2,3-dimethylpentane. Draw the equation for this reaction. Show the stereochemistry of the reactant and the product.
Sight along the C2-Cl bond of 2-methylpropane (isobutane).
a. Draw a Newman projection of the most stable conformation.
b. Draw a Newman projection of the least stable conformation.
c. Make a graph of energy versus angle of rotation around the C2-Cl bond.
d. Assign relative values to the maxima and minima in your graph, given that an H↔H eclipsing interaction costs 0 kJ/mol and an H↔CH3 eclipsing interaction costs 6.0 kJ/mol.
Chapter 5 Solutions
Organic Chemistry - Standalone book
Ch. 5.1 - Prob. 1PCh. 5.1 - Prob. 2PCh. 5.1 - Many compounds contain more than one functional...Ch. 5.2 - Prob. 4PCh. 5.3 - Prob. 5PCh. 5.4 - Classify the isomeric C4H10O alcohols as being...Ch. 5.5 - Bromine is less electronegative than chlorine, yet...Ch. 5.6 - Prob. 8PCh. 5.7 - Prob. 9PCh. 5.8 - Prob. 10P
Ch. 5.8 - Prob. 11PCh. 5.9 - Carbocations are key intermediates in petroleum...Ch. 5.9 - Prob. 13PCh. 5.9 - Prob. 14PCh. 5.11 - Prob. 15PCh. 5.13 - Prob. 16PCh. 5.14 - For the reaction of a primary alcohol RCH2OH with...Ch. 5.15 - Prob. 18PCh. 5 - Write structural formulas for each of the...Ch. 5 - Prob. 20PCh. 5 - Prob. 21PCh. 5 - Write structural formulas for all the...Ch. 5 - Prob. 23PCh. 5 - Prob. 24PCh. 5 - Epichlorohydrin is the common name of an...Ch. 5 - Prob. 26PCh. 5 - Prob. 27PCh. 5 - Prob. 28PCh. 5 - Some of the most important organic compounds in...Ch. 5 - Prob. 30PCh. 5 - Prob. 31PCh. 5 - Prob. 32PCh. 5 - Prob. 33PCh. 5 - Prob. 34PCh. 5 - Prob. 35PCh. 5 - Prob. 36PCh. 5 - Prob. 37PCh. 5 - Prob. 38PCh. 5 - Prob. 39PCh. 5 - Prob. 40PCh. 5 - The reaction of 2,2-dimethyl-1-propanol...Ch. 5 - (a) Assuming that the rate-determining elementary...Ch. 5 - The reaction of 3-tert-butyl-3-pentanol with...Ch. 5 - Prob. 44PCh. 5 - Prob. 45PCh. 5 - Prob. 46DSPCh. 5 - Prob. 47DSPCh. 5 - Prob. 48DSPCh. 5 - Prob. 49DSPCh. 5 - Prob. 50DSPCh. 5 - Prob. 51DSPCh. 5 - Prob. 52DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Alcohols undergo an oxidation reaction to yield carbonyl compounds on treatment with CrO3. For example, 2-tert-butylcyclohexanol gives 2-tert-butylcyclohexanone. If axial OH groups are generally more reactive than their equatorial isomers, which do you think reacts faster, the cis isomer of 2-tert-butylcyclohexanol or the trans isomer? Explain.arrow_forwardDraw the products of the following reactions. If the products can exist as stereoisomers show what stereoisomers are formed. a. cis-2-pentene + Br2/CH2Cl2 b. trans-2-pentene + Br2/CH2Cl2 c. 1- butene + HCl d. methylcyclohexene + HBr e. trans-3-hexene + Br2/CH2Cl2 f. cis-3-hexene + Br2/CH2Cl2 g. 3,3-dimethyl-1-pentene + HBr h. cis-2-butene + HBr i. (Z)-2,3-dichloro-2-butene + H2, Pd/C j. (E)-2,3-dichloro-2-butene + H2, Pd/C k. (Z)-3,4-dimethyl-3-hexene + H2, Pd/C l. (E)-3,4-dimethyl-3-hexene + H2, Pd/Carrow_forward(a) Write the structures of the following compounds and mark them as chiral or achiral. 4 (i) 2-Bromopentane (ii) 3-Bromopentane (iii) 1-Bromo-2-methylbutane (iv) 2-Chloro-3-methylbutane (b) Identify the asymmetric carbon in the chiral compounds. (c) Write the structure of the other enantiomer of the chiral compounds.arrow_forward
- How many tetrahedral stereogenic centers does PGF2α contain? Draw its enantiomer. How many of its double bonds can exhibit cis-trans isomerism? Considering both its double bonds and its tetrahedral stereogenic centers, how many stereoisomers are possible for PGF2α?arrow_forwardDraw the products of the following reactions. If the products can exist as stereoisomers, show which stereoisomers are formed.a. cis-2-pentene + HClb. trans-2-pentene + HClc. 1-ethylcyclohexene + H2O + H2SO4d. 2,3-dimethyl-3-hexene + H2, Pd/Ce. 1,2-dimethylcyclohexene + HCl f. 1,2-dideuteriocyclohexene + H2, Pd/Cg. 3,3-dimethyl-1-pentene + Br2/CH2Cl2h. 1E2@3,4@dimethyl@3@heptene + H2, Pd/Ci. 1Z2@3,4@dimethyl@3@heptene + H2, Pd/Cj. 1@chloro@2@ethylcyclohexene + H2, Pd/Carrow_forwardBASED ON THE FIGURE WHAT IS 1. The least stable conformation of 1-bromo-3-ethylcyclohexane. 2. The most stable conformation of 1-bromo-3-ethylcyclohexane. 3. The structure that has four 1,3-diaxial interactions. 4.arrow_forward
- Organic Chemistry Indicate which carbocation is the most stable carbocation and which carbocation is the least stable carbocation?Çok Satırlı Metin.arrow_forward4. Write all possible pairs of Enantiomers and Diastereomers from the following molecules.(use numbers(1;1:1;:V) writing pairs) A) 1. 2R,3R-dichloropentane II. 2R,3S-diChloropentane II. 2S,3R-dichloropentane IV.2S,3S-dichloropentane Enantiomers Diastereomers B) I. 3S,4S-dimethyhexane II. 3S,4R-dimethyhexane III. 3R,4S-dimethyhexane IV. 3R,4R-dimethyhexane Enantiomers Diastereomersarrow_forwardHow many tetrahedral stereogenic centers does PGF2α contain? Draw its enantiomer. How many of its double bonds can exhibit cis-trans isomerism? Considering both its double bonds and its tetrahedral stereogenic centers, how many stereoisomers are possible for PGFα?arrow_forward
- Draw the structure of each molecule below and put an asterisk on each tetrahedral stereocenter. i) 3,4-dichlorohexane ii) 3-bromo-1-iodo-5-methylhexane iii) 3-bromo-2,4-dimethylpentanearrow_forward3,4-Dimethylpent-1-ene has the formula CH2“CH¬CH(CH3)¬CH(CH3)2. When pure (R)-3,4-dimethylpent-1-ene is treated with hydrogen over a platinum catalyst, the product is (S)-2,3-dimethylpentane. Has the chiral center retained its configuration during this hydrogenation, or has it been inverted?arrow_forwardWhen HBr adds across the double bond of 1,2-dimethylcyclopentene, the product is a mixture of the cis and trans isomers. Show why this addition is not stereospecific.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License