
Concept explainers
Determine the number of nodes along the internuclearaxis for each of the
Interpretation: The number of nodes along the internuclear axis for each of the
Concept introduction: Born-Oppenheimer approximation state that nuclei are heavier than electrons that considered fixed in space whereas the electrons move constantly around them. The Born−Oppenheimer approximation solves the electronic Schrodinger equation for
Answer to Problem 1P
The number of nodes in
Explanation of Solution
Node is point in molecular orbitals where probability to find the electron density is zero. Nodal plane is the imaginary plane where probability to find electrons is zero.
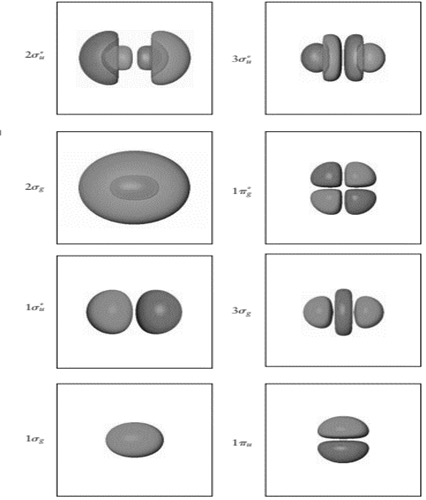
In the figure, node is the oval region. It is formed when plane white surface cut the spherical orbital into two equal halves having opposite symmetry. Also, there are six
In
In
In
In
In
In
Therefore, number of nodes in
Want to see more full solutions like this?
Chapter 6 Solutions
Principles of Modern Chemistry
- Considering only the molecular orbitals formed by combinations of the 2s atomic orbitals, how many molecular orbitals can be formed by 1000 Li atoms? In the lowest energy state, how many of these orbitals will be populated by pairs of electrons and how many will be empty?arrow_forwardUse MO theory to predict the bond order and the number of unpaired electrons in the super-oxide ion, , and the peroxide ion, .arrow_forwardPredict whether the MO diagram S2 would show s-p mixing or not.arrow_forward
- Based on the discussion so far, identify a characteristic that is common to all situations where electron-region geometry and molecular geometry are the same for a molecule or a polyatomic ion.arrow_forwardDetermine if the following species have permanent dipole moments. a The carbonate ion, CO32 b The phosphate ion, PO43 c Uranium hexafluoride, UF6 d Bromine, Br2.arrow_forwardMolecular Orbital Theory (See Examples 9.49.6.) The hydrogen molecular ion, H2+, can be detected spectroscopically. Write the electron configuration of the ion in molecular orbital terms. What is the bond order of the ion? Is the hydrogenhydrogen bond stronger or weaker in H2+ than in H2s?arrow_forward
- True or false: Boron contains 2s22p1 valence electrons, so only one p orbital is needed to form molecular orbitals.arrow_forwardIn the molecular orbital mode l, compare and contrast bonds with bonds. What orbitals form the bonds and what orbitals form the bonds? Assume the z-axis is the internuclear axis.arrow_forwardHow many molecular orbitals can be constructed from a diatomic molecule in which s. p, d. and f orbitals are all important for bonding?arrow_forward
- Which of the following molecules and ions have a bond order of ½: H2, H2 + , H2 – , and He2 2+? Please Sketch the MO diagrams to prove your answer.arrow_forwardConstruct a Frost circle for a planar eight-membered ring with one 2p orbital on each atom of the ring and show the relative energies of its eight π molecular orbitals. Which are bonding MOs, which are antibonding, and which are nonbonding?arrow_forwardA Molecular Orbital can hold a maximum of how many electrons? 0 1 2 as many e- as the molecule hasarrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





