
Concept explainers
Interpretation:
The IUPAC names of the given compounds are to be determined.
Concept introduction:
The IUPAC name of
The longest carbon chain is numbered in a way so as to give a low numbering to the carbon with the halogen atom.
The substituents on the parent alkane are written alphabetically before the name of the parent alkane.
In cyclic compounds, the prefix ‘cyclo’ is used before the name of an alkane.
Substituents present on the same side of a ring are named with the prefix cis while those on opposite sides are named with the prefix‘trans’.
Answer to Problem 1PP
Solution:
Explanation of Solution
a)
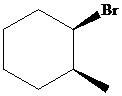
The parent alkane here is cyclohexane. The substituents are a bromine atom and a methyl group. Number the cyclohexane ring starting from the carbonwithbromine atom.
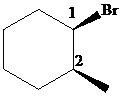
Putting the names of the substituents as the prefix alphabetically along with their positions before the name of the parent alkane, the IUPAC name can be written as
The two substituents are on the same side. Hence, the complete IUPAC name of the compound is
b)

The parent alkane here is cyclohexane. The substituents are a bromine atom and a methyl group. Number the cyclohexane ring starting from the carbon with the bromine atom. Both the bromine group and the methyl group are in equatorial position.
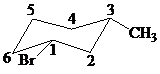
Putting the names of substituents as the prefix alphabetically along with their positions before the name of the parent alkane, the name can be written as
The two substituents are equatorial at the
Hence, the complete IUPAC name of the compound is
c)
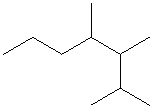
Select the longest continuous carbon chain and number it from that end, giving the lower number to the substituents.
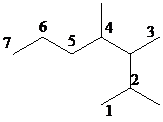
The parent chain has seven carbon atoms. Thus, the parent alkane is heptane. There are three methyl substituents at
Hence, the IUPAC name of the compound is
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry
- Write an acceptable IUPAC name for the compound below. Include 'cis' and trans' as part of the name when stereochemistry is illustrated.arrow_forwardGive IUPAC name of the following molecule and indicate Z or E. Answer all four of themarrow_forwardWrite an acceptable IUPAC name for the compounds below. Include 'cis' and trans' as part of the name when stereochemistry is illustrated.arrow_forward
- Using the information provided below, deduce the identity of the compound I, II, III, IV and V. What is the IUPAC name of compound I, II, III, IV and V?arrow_forwardGive the reagents and conditions necessary for the following conversion. A to B, B to D, B to C, B to E, E to F, E to G, G to H, H to I. Hence deduce the name and structural formula of the compounds C and I. Compare the procedure for converting F and E to G.arrow_forwardWrite a structural formula and give two acceptable IUPAC names for each alkene of molecular formula C7H14 that has a tetrasubstituted double bond.arrow_forward
- give the structural formula for compounds A to Garrow_forwardCompound F may be synthesised by the method attached: Draw the structural formulas of compounds A, C, D, E and F in the boxes providedarrow_forwardWrite down the explicit structures of A, B, C and D in accordance with the reactions given in the synthesis plan, which shows the synthesis of D, a pheromone of moth.arrow_forward
- Provide the IUPAC name of the compound shown below please don’t forget to include the stereocenters.arrow_forwardGive the IUPAC name for the following compound. Be sure to indicate stereochemistry where necessary.arrow_forwardWrite the IUPAC name of the following molecule: *Lowercase letters only and DO NOT put space in between. If there is a complex substituent, name it SYSTEMATICALLY. Do not use special names. DO NOT include stereochemistry.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





