
Interpretation:
The reaction which occurs faster in each of the given reactions is to be determined and the reason is to be explained.
Concept introduction:
Alkyl iodides are several times more reactive than alkyl bromides. These reactivity differences can be related to the carbon-halogen bond strength and the basicity of the halide anion. Alkyl iodides have the weakest carbon-halogen bond and require the lowest activation energy to break.
Regarding basicity of the halide leaving the group, iodide is the weakest base. Generally, it is true that, the less basic the leaving group, the smaller the energy requirement for cleaving its bond to carbon and the faster the rate.
Alkyl groups that are adjacent to the carbon atom to the point of nucleophilic attack decrease the rate of the
Protic solvents having
In
Answer to Problem 27P
Solution:
a)
b)
c) Cyclohexyl chloride reacts faster than hexyl chloride by the
d) Tert-butyl bromide reacts faster than
e) sec-butyl bromide reacts faster than isobutyl bromide by the
f) The reaction of
g) The reaction of
Explanation of Solution
a)
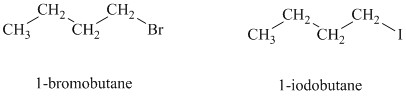
Both the given
b)
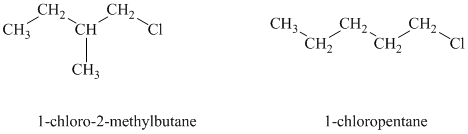
Both the given alkyl halides are primary alkyl halides. The reagent is sodium iodide in acetone. Both these suggest an
c)
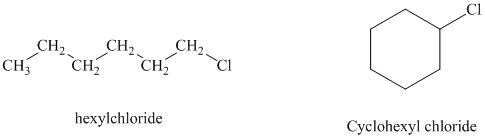
Hexyl chloride is a primary alkyl halide whereas cyclohexyl chloride is a secondary alkyl halide. The solvent is ethanol, which is a polar protic solvent. The nucleophile is the azide ion, which is a good nucleophile. Solvation of the azide ion by ethanol reduces the rate of bimolecular substitution. Polar protic solvents favor
d)

The solvent is ethanol, which is a polar protic solvent. It favors the
e)
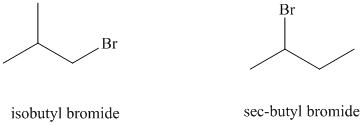
The solvent is aqueous formic acid, which is a polar protic solvent. It favors the
f)
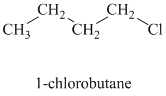
As long as the nucleophilic atom is the same, the more basic the nucleophile, the more reactive it is. The methoxide ion is more basic and more nucleophilic than the acetate ion. Thus, the reaction of
g)
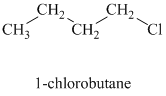
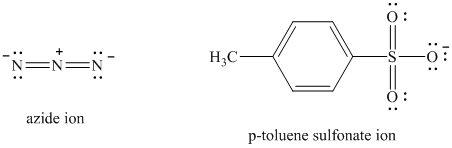
Comparing the nucleophilic atoms, the azide ion is more nucleophilic than the p-toluene sulfonate ion since nitrogen is less electronegative than oxygen. Thus, the reaction of
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry - Standalone book
- Write the main product of the following reaction and say if the reaction has gone through an SN1, SN2, E1 or E2 mechanism. Explain it. 2-Chloro-2-methylbutane with water.arrow_forwardMarkovnikov’s Rule is required in order to predict that the major substrate product in the reaction between HCl (hydrochloric acid) and ___ will be ___ . benzene; chlorobenzene 1-butene; 1-chlorobutane 1-hexene; 2-chlorohexane 3 of these 4 responses are correct 1-octene; 2-bromooctanearrow_forwardExplain why the ether obtained by treating an optically active alcohol with PBr3 in pyridine followed by sodium methoxide has the same configuration as the alcohol, whereas the ether obtained by treating the alcohol with tosyl chloride followed by sodium methoxide has a configuration opposite that of the alcohol.arrow_forward
- The base-promoted rearrangement of an -haloketone to a carboxylic acid, known as the Favorskii rearrangement, is illustrated by the conversion of 2-chlorocyclohexanone to cyclopentanecarboxylic acid. It is proposed that NaOH first converts the a-haloketone to the substituted cyclopropanone shown in brackets and then to the sodium salt of cyclopentanecarboxylic acid. (a) Propose a mechanism for base-promoted conversion of 2-chlorocyclohexanone to the proposed intermediate. (b) Propose a mechanism for base-promoted conversion of the proposed intermediate to sodium cyclopentanecarboxylate.arrow_forwardShow reagents to convert bromocyclopentane to each of the following compounds.arrow_forwardWittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forward
- Fill in the appropriate reagent or starting material in each of the following reactions.arrow_forwardwe know that ethers, such as diethyl ether and tetrahydrofuran, are quite resistant to the action of dilute acids and require hot concentrated HI or HBr for cleavage. However, acetals in which two ether groups are linked to the same carbon undergo hydrolysis readily, even in dilute aqueous acid. How do you account for this marked difference in chemical reactivity toward dilute aqueous acid between ethers and acetals?arrow_forwardWhen 1,2-cyclohexanediol is dehydrated in the presence of concentrated sulfuric acid, the major product is not an alkene. Instead, you get cyclohexanone. Write a reasonable and detailed mechanism for the dehydration of 1,2-cyclohexanediol to form cyclohexanone. Use curved arrows to show the flow of electrons and draw the structures of all intermediates and byproducts formed in the course of this reaction as well as any alternative resonance structures that will help you to account for the formation of the major product observed in this reaction.arrow_forward
- Give the product(s) that would be formed when 3-methyl-3-pentanol is subjected to acid-catalyzed dehydration. If more than one product would be formed, designate the one that would be the major product.arrow_forwardWrite the etherification reactions of isopropyl bromide and 2-bromo-1-nitropropane with phenol . Which reaction takes place faster and why?arrow_forwardPlease be clear in your writing, solve step by step Compound A (C9H12), when hydrogenated by catalysis on Pd / C, absorbs 3 equivalents of H2 to give compound B (C9H18). Ozonolysis of compound A gives cyclohexanone (C6H10O). Compound A reacting with NaNH2 / NH3 followed by addition of CH3Br gave compound C (C10H14). What are the structures of compounds A, B, and C?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

