
Chemistry In Focus
7th Edition
ISBN: 9781337399692
Author: Tro, Nivaldo J.
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6, Problem 2SC
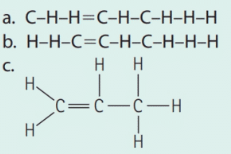
Which structure corresponds to
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 6 Solutions
Chemistry In Focus
Ch. 6 - Drawing Structural and Condensed Structural...Ch. 6 - Prob. 6.2YTCh. 6 - Drawing Structural Formulas for Isomers Draw...Ch. 6 - Prob. 6.4YTCh. 6 - Prob. 1SCCh. 6 - Which structure corresponds to CH2=CHCH3?Ch. 6 - Prob. 3SCCh. 6 - What property is characteristic of chlorinated...Ch. 6 - Prob. 5SCCh. 6 - Prob. 6SC
Ch. 6 - Prob. 7SCCh. 6 - Prob. 1ECh. 6 - Prob. 2ECh. 6 - Prob. 3ECh. 6 - Prob. 4ECh. 6 - What is vitalism? Why did vitalism become a...Ch. 6 - Prob. 6ECh. 6 - Prob. 7ECh. 6 - Prob. 8ECh. 6 - Prob. 9ECh. 6 - List four common fuels used by our society, and...Ch. 6 - Prob. 11ECh. 6 - Why are alkenes and alkynes called unsaturated...Ch. 6 - Prob. 13ECh. 6 - Prob. 14ECh. 6 - Prob. 15ECh. 6 - Prob. 16ECh. 6 - Prob. 17ECh. 6 - Prob. 18ECh. 6 - Prob. 19ECh. 6 - Prob. 20ECh. 6 - Prob. 21ECh. 6 - Prob. 22ECh. 6 - Prob. 23ECh. 6 - Prob. 24ECh. 6 - Prob. 25ECh. 6 - Prob. 26ECh. 6 - Prob. 27ECh. 6 - Prob. 28ECh. 6 - Prob. 29ECh. 6 - Prob. 30ECh. 6 - Prob. 31ECh. 6 - Prob. 32ECh. 6 - Prob. 33ECh. 6 - Prob. 34ECh. 6 - Prob. 35ECh. 6 - Prob. 36ECh. 6 - Prob. 37ECh. 6 - Prob. 38ECh. 6 - Prob. 39ECh. 6 - Prob. 40ECh. 6 - Prob. 41ECh. 6 - Prob. 42ECh. 6 - Naming Hydrocarbons Name each alkane:Ch. 6 - Name each alkane:Ch. 6 - Name each alkyne:Ch. 6 - Name each alkyne: a.CH3CHCHCH2CH2CH3Ch. 6 - Name each alkyne:Ch. 6 - Prob. 48ECh. 6 - Prob. 49ECh. 6 - Prob. 50ECh. 6 - Drawing Hydrocarbon Structures from Names Draw the...Ch. 6 - Draw the condensed structural formula for each...Ch. 6 - Prob. 53ECh. 6 - Prob. 54ECh. 6 - Prob. 55ECh. 6 - Prob. 56ECh. 6 - Functionalized Hydrocarbons Identify each compound...Ch. 6 - Identify each compound according to its functional...Ch. 6 - Identify each compound according to its functional...Ch. 6 - Identify each compound according to its functional...Ch. 6 - Propane, CH3CH2CH3, is a gas at room temperature,...Ch. 6 - Prob. 62ECh. 6 - What was the impact of vitalisms downfall on...Ch. 6 - Why do you think our society has mixed feelings...Ch. 6 - Prob. 65ECh. 6 - Prob. 66ECh. 6 - Prob. 67ECh. 6 - Any one molecule can be represented many ways. For...Ch. 6 - Explain why the formula CH3CH2CH3 cannot mean:...Ch. 6 - Prob. 70ECh. 6 - Prob. 71ECh. 6 - Prob. 72ECh. 6 - Prob. 73E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY