
Interpretation:
From the given experimental data both in absence and presence of its inhibitor a Lineweaver–Burk plot is to be drawn and values of
Concept introduction:
An inhibitor may be defined as a molecule that binds with the enzymes and decreases the activity of enzyme toward the substrate and this decrease in enzyme activity is called enzymatic inhibition.
Enzymatic inhibition can be of many types. When substrate and inhibitor of an enzyme compete with each other to bind with the enzyme and both of them are structurally same it is known as competitive inhibition. On the other hand, when inhibitors don’t compete with the substrate and both of them can bind to the enzyme at their specific site present on the substrate it is known as noncompetitive inhibition.
To determine the rate of the reaction, experimental data must be put into Lineweaver Burk plot where the x-axis represents the values of
Answer to Problem 60RE
Plot the reciprocal of substrate concentration along the x-axis and the reciprocal of velocities (both in the presence and absence of inhibitors) along the y-axis.
Before drawing the plot, the data needs to be modified so that it can be put into Lineweaver–Burk plot. The modification of the data is
The Lineweaver–Burk plot from above data is as follows:
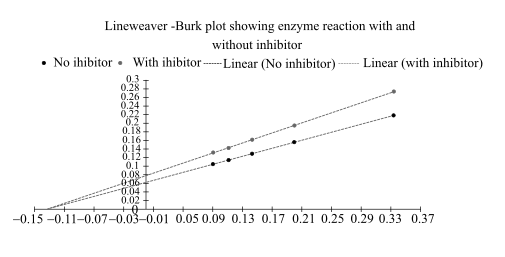
From this plot,
Or,
The value
So, the value of
The
The value of
By comparing these two plots it can be said that it is a type of noncompetitive inhibition.
Explanation of Solution
In the case of competitive inhibition, the substrate and inhibitor compete with each other to bind with the active site of the enzyme. By increasing substrate concentration, the effect of competitive inhibition can be discarded. In high substrate concentration
In the case of non-competitive inhibition, inhibitors bind with the allosteric site of the enzyme and enzyme itself can bind with both substrate and inhibitors. It is possible because it has two different sites for both inhibitor and substrate. When the inhibitor binds with an enzyme it blocks its activity even if the substrate is also bound to the enzyme. These inhibitors do not change the substrate affinity of the enzyme so the
In this Lineweaver Burk plot,
The
Want to see more full solutions like this?
Chapter 6 Solutions
Biochemistry
- MATHEMATICAL Consider the reaction AB+C, where G=0.00. (a) What is the value of G (not G) when the initial concentrations of A, B, and C are 1 M, 103M,and106M? (b) Try the same calculations for the reaction D+EF, for the same relative order of concentrations. (c) Try the same calculations for the reaction GH, if the concentrations are 1Mand103M for G and H, respectively.arrow_forwardREFLECT AND APPLY A model is proposed to explain the reaction catalyzed by an enzyme. Experimentally obtained rate data fit the model to within experimental error. Do these findings prove the model?arrow_forwardREFLECT AND APPLY When we compare the binding of I and of S to the enzyme in a mixed noncompetitive inhibitor, we assumed that the binding of I decreased the affinity of the enzyme for S. What would happen if the opposite were true?arrow_forward
- REFLECT AND APPLY Comment on the energetics of protein folding in light of the information in this chapter.arrow_forwardMATHEMATICAL For an enzyme that displays MichaelisMenten kinetics, what is the reaction velocity, V (as a percentage of Vmax), observed at the following values? (a) [S]=KM (b) [S]=0.5KM (c) [S]=0.1KM (d) [S]=2KM (e) [S]=10KMarrow_forwardREFLECT AND APPLY Would nature rely on the same enzyme to catalyze a reaction either way (forward or backward) if the DG were 0.8kcalmol1? If it were 5.3kcalmol1?arrow_forward
- MATHEMATICAL If a reaction can be written AB, and the G is 20kJmol1, what would the substrate/product ratio have to be for the reaction to be thermodynamically favorable?arrow_forwardREFLECT AND APPLY What is the relationship between a transition-state analog and the induced-fit model of enzyme kinetics?arrow_forwardREFLECT AND APPLY Noncompetitive inhibition is a limiting case in which the effect of binding inhibitor has no effect on the affinity for the substrate and vice versa. Suggest what a LineweaverBurk plot would look like for an inhibitor that had a reaction scheme similar to that on page 159 (noncompetitive inhibition reaction), but where binding inhibitor lowered the affinity of EI for the substrate.arrow_forward
- REFLECT AND APPLY Suggest a reason why heating a solution containing an enzyme markedly decreases its activity. Why is the decrease of activity frequently much less when the solution contains high concentrations of the substrate?arrow_forwardREFLECT AND APPLY The enzyme D-amino acid oxidase has a very high turnover number because the D-amino acids are potentially toxic. The KM for the enzyme is in the range of 1 to 2 mM for the aromatic amino acids and in the range of 15 to 20 mM for such amino acids as serine, alanine, and the acidic amino acids. Which of these amino acids are the preferred substrates for the enzyme?arrow_forwardREFLECT AND APPLY Would you expect the structure of a non- competitive inhibitor of a given enzyme to be similar to that of its substrate?arrow_forward
 BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
