
(a)
Interpretation:
The conversion after
Concept Introduction:
The conversion, X can be defined as the moles of any species A that are reacted per mole of A fed in the reactor.
A batch reactor is a closed system with no continuous flow of reactants entering the system or products leaving the system while the reaction takes place.
In most batch reactors, the longer a reactant stays in the reactor, the more the reactant is converted to product until either equilibrium is reached or the reactant is exhausted. Consequently, in batch systems the conversion X is a function of the time the reactants spend in the reactor.
(a)
Answer to Problem 6.3P
The conversion after
Explanation of Solution
The given second-order liquid phase reaction which takes place in a batch reactor is as follows.
The given specific rate constant for the reaction is
The given volume of reactor 1 is
The concentration of each reactant after the mixing is
The temperature of the batch reactor is
The given two reactors are shown below.
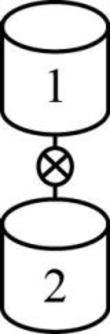
Figure 1
The concentration of the reactant is assumed to be
Where,
The expression for the integrated general concentration of the reactant before mixing the
Where,
Substitute the value of
Substitute
The expression to calculate the conversion after
Substitute
For the conversion after
Substitute
For the conversion after
Substitute
Therefore, the conversion after
(b)
Interpretation:
The conversion and concentration of each species present in the reactor 1 after
Concept Introduction:
The conversion, X can be defined as the moles of any species A that are reacted per mole of A fed in the reactor.
A batch reactor is a closed system with no continuous flow of reactants entering the system or products leaving the system while the reaction takes place.
In most batch reactors, the longer a reactant stays in the reactor, the more the reactant is converted to product until either equilibrium is reached or the reactant is exhausted. Consequently, in batch systems the conversion X is a function of the time the reactants spend in the reactor.
(b)
Answer to Problem 6.3P
The concentration after 10,
Explanation of Solution
The given second-order liquid phase reaction which takes place in a batch reactor is as follows.
The given specific rate constant for the reaction is
The given volume of reactor 1 is
The concentration of each reactant after the mixing is
The temperature of the batch reactor is
The given two reactors are shown below.
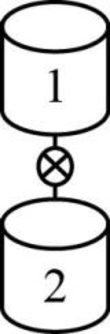
Figure 1
It is given that after 10 minutes, the species in the reactor 1 are drained out at the rate of
The solution that is left out in 10 minutes is
The total solution that is remaining in the reactor 1 is
If the solution is
So, in
The concentration of each species is calculated below given below.
Thus, the concentration of each species remains the same in
So, the concentration of each species after
The concentration of the product after
So, the concentration of each species after
The concentration of the product after
The expression to calculate the conversion after
Substitute
Substitute
For the conversion after
The concentration of the product after
Therefore, the concentration after
Substitute
Therefore, the conversion after
(c)
Interpretation:
The conversion and concentration of each species present in the reactor 2 that is filling up from reactor 1 after
Concept Introduction:
The conversion, X can be defined as the moles of any species A that are reacted per mole of A fed in the reactor.
A batch reactor is a closed system with no continuous flow of reactants entering the system or products leaving the system while the reaction takes place.
In most batch reactors, the longer a reactant stays in the reactor, the more the reactant is converted to product until either equilibrium is reached or the reactant is exhausted. Consequently, in batch systems the conversion X is a function of the time the reactants spend in the reactor.
(c)
Answer to Problem 6.3P
The conversion of each species present in the reactor 2 that is filling up from reactor 1 after
Explanation of Solution
The given second-order liquid phase reaction which takes place in a batch reactor is as follows.
The given specific rate constant for the reaction is
The given volume of reactor 1 is
The concentration of each reactant after the mixing is
The temperature of the batch reactor is
The given two reactors are shown below.
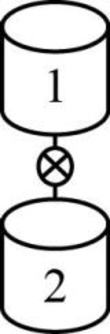
Figure 1
It is given that after 10 minutes, the species in the reactor 1 are drained out at the rate of
The solution that is left in 10 minutes is
The total solution that is remaining in the reactor 1 is
If the solution is
So, in
The concentration of each species is calculated below given below.
Thus, the concentration of each species remains the same in
So, the concentration of each species after
The concentration of the product after
At the end of 50 minutes, the volume of reactor 1 is
The concentration of the product after
At the end of 50 minutes, the volume of reactor 2 has
Therefore, the concentration after
Therefore, the conversion after
(d)
Interpretation:
The overall conversion and concentration when the contents of the reactants are mixed together at the end of
Concept Introduction:
The conversion, X can be defined as the moles of any species A that are reacted per mole of A fed in the reactor.
A batch reactor is a closed system with no continuous flow of reactants entering the system or products leaving the system while the reaction takes place.
In most batch reactors, the longer a reactant stays in the reactor, the more the reactant is converted to product until either equilibrium is reached or the reactant is exhausted. Consequently, in batch systems the conversion X is a function of the time the reactants spend in the reactor.
(d)
Answer to Problem 6.3P
The overall conversion of the two reactor after mixing the contents is
Explanation of Solution
The given second-order liquid phase reaction which takes place in a batch reactor is as follows.
The given specific rate constant for the reaction is
The given volume of reactor 1 is
The concentration of each reactant after the mixing is
The temperature of the batch reactor is
The given two reactors are shown below.
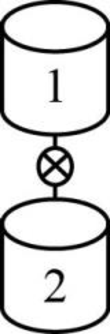
Figure 1
At the end of 50 minutes, the volume of reactor 1 is
The concentration of the product after
The number of moles of reactant are
The number of moles of the product are
At the end of 50 minutes, the volume of reactor 2 is
So, the total volume after the mixing of the species of reactor 1 and 2 is
The total moles of reactants after the mixing of the species of reactor 1 and 2 is
The total concentration of reactants after the mixing of the species of reactor 1 and 2 is
The overall conversion after mixing in reactor,
The total moles of product after the mixing of the species of reactor 1 and 2 is
The total concentration of product after the mixing of the species of reactor 1 and 2 is
The conversion,
Therefore, the overall conversion of the two reactor after mixing the contents is
(e)
Interpretation:
The application of the six ideas that is given in table on this problem is to be stated.
Concept Introduction:
The conversion, X can be defined as the moles of any species A that are reacted per mole of A fed in the reactor.
The reactors that consist of the particles of solid catalysts which are packed in the form of bed are known as fixed bed reactors.
(e)
Answer to Problem 6.3P
The application of the six ideas that is given in table on this problem is not appropriate.
Explanation of Solution
The given six ideas are as follows.
- Brainstorm ideas to ask another question or advice another calculation for the homework problem.
- Brainstorm ways to work this homework problem incorrectly.
- Brainstorm ways to make this problem highly easy or more difficult.
- Brainstorm a list of things that can be learned from working this homework problem.
- Brainstorm the reason why the calculations over predicted the conversion that measured if the reactor was kept on stream.
- The “What if…” questions are particularly effective when they are used with the living example problems in which variation in parameters to explore the problem is carried out.
These six ideas are not relevant according to the demand of the question. Thus, The application of the six ideas that is given in table on this problem cannot be possible according to the demand of the question.
Want to see more full solutions like this?
Chapter 6 Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





