
Interpretation:
Each line in the phase diagram, in terms of the derivative it represents, is to be labeled.
Concept introduction:
Phase diagram represents the different physical states of a substance at different values of temperature and pressure. In the water, the molar volume of solid is greater than the molar volume of the liquid.
Answer to Problem 6.66E
The phase transition between solid phase to liquid phase is represented by the derivative,
The phase transition between solid phase to liquid phase is represented by the derivative,
The phase transition between solid phase to liquid phase is represented by the derivative,
The phase transition between solid phases is represented by the derivative,
Explanation of Solution
The phase diagram of water shown in Figure 6.6 is shown below.
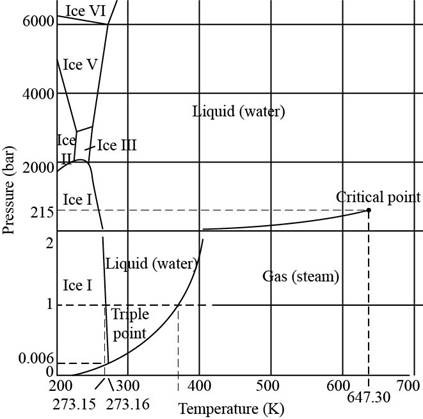
Figure 1
In the given diagram, the total numbers of phase transitions represented are,
1. Gas phase to liquid phase
2. Gas phase to ice phase
3. Liquid phase to ice phase
4. Liquid phase to ice phase
5. Liquid phase to ice phase
6. Liquid phase to ice phase
7. Ice phase
8. Ice phase
9. Ice phase
10. Ice phase
11. Ice phase
12. Ice phase
Thus, the total number of phase transitions are
The phase transition between solid phase to liquid phase, that is, ice phase
Where,
•
•
The phase transition between gas phase to ice phase
Where,
•
•
The volume of gas is much greater than the volume of solid. Therefore, it can be neglected.
The phase transition between solid phase and liquid phase is represented by the derivative,
Where,
•
•
The volume of gas is much greater than the volume of liquid. Therefore, it can be neglected.
The phase transition between solid phases is represented by the derivative,
Where,
•
•
The phase transition between solid phase to liquid phase is represented by the derivative,
The phase transition between solid phase to liquid phase is represented by the derivative,
The phase transition between solid phase to liquid phase is represented by the derivative
The phase transition between solid phases is represented by the derivative,
Want to see more full solutions like this?
Chapter 6 Solutions
Physical Chemistry
- Use the phase diagram of water in Figure 6.6 and count the total number of phase transitions that are represented.arrow_forward6.9. Identify and explain the sign on in equation 6.5 if it used for (a) a solid-to-gas phase transition (sublimation), (b) a gas-to-liquid phase transition (condensation).arrow_forwardThe phase diagram for a substance, X, is shown below. At a given temperature and pressure liquid X is in equilibrium with its vapour inside a closed, thermally insulated vessel, as shown by point A on the phase diagram. The vessel is connected to another identical vessel, which is under vacuum. Describe what happens when the stop-cock between the two vessels is opened.arrow_forward
- The next phase diagram looks at the effect of changing pressure at constant temperature. Name and explain the significance of the point C.arrow_forwardFor the phase diagram given, match the labels for:arrow_forwardThe temperature in space is about 3.00 K. If the normal boiling point of water is 100.0°C, what would the vapour pressure be in space? (you can, for the purposes of this question, ignore that it would freeze)arrow_forward
- What is the vapor pressure of Ag at 2365.8 K if its heat of vaporization and boiling point are 285.00 kJ/mol and 2485.2 K, respectively?arrow_forwardWhat is represented by point D on the phase diagram? (Note: C is incorrect)arrow_forwardgive 5 examples of a 1 phase diagram and show its phase rulearrow_forward
- Does water density appear to be more sensitive to a change in temperature at point A or point B?arrow_forwardFrom the following phase diagram,give the nature of phases present in the numbered areas and its the degrees of freedom. marrow_forwardRefer to Fig. 4A.8. Which phase or phases would you expect to be present for a sample of CO2 at: (i) 200 K and 2.5 atm; (ii) 300 K and 4 atm; (iii) 310 K and 50 atm?arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


