
Concept explainers
Draw Lewis structures for the following four isoelectronic species: (a) CO, (b) NO+, (c) CN−, (d) N2. Show formal charges. (See Problem 6.69.)
(a)
Interpretation: The Lewis structures for the given isoelectronic species should be shown.
Concept Introduction:
- Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
- It is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
To find: The Lewis structure for the given set of isoelectronic species.
Answer to Problem 6.83QP

Explanation of Solution
Given isoelectronic species is below.
Lewis structure of the above isoelectronic species is drawn below.

The total number of valence electrons is found to be 10, where carbon and oxygen has 4 and 6 valence electrons respectively.
The 8 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on nitrogen atom to complete the octet.
Since the octets of carbon atoms are not filled, a triple bond was made between carbon and oxygen atoms in expense of two electrons where the remaining four electrons are distributed equally over two atoms present in the given species.
(b)
Interpretation: The Lewis structures and the formal charges for the given isoelectronic species should be shown.
Concept Introduction:
- Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
- It is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
To find: The Lewis structure for the given set of isoelectronic species.
Answer to Problem 6.83QP
Explanation of Solution
Given isoelectronic species is below.
Lewis structure of the above isoelectronic species is drawn below.

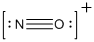
The total number of valence electrons is found to be 11, where nitrogen and oxygen contains 5 and 6 valence electrons respectively. The whole charge of the molecule is +1 that results in the total number of valence electrons as 10.
The 8 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on nitrogen atom to complete the octet.
Since the octets of nitrogen atoms are not filled, a triple bond is made between nitrogen and oxygen atoms in expense of two electrons where the remaining four electrons are distributed over the 2 atoms present in the given molecule.
(c)
Interpretation: The Lewis structures and the formal charges for the given isoelectronic species should be shown.
Concept Introduction:
- Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
- It is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
To find: The Lewis structure for the given set of isoelectronic species.
Answer to Problem 6.83QP

Explanation of Solution
Given isoelectronic molecule is below.
Lewis structure of the above isoelectronic species is drawn below.

The total number of valence electrons is found to be 9, where nitrogen and carbon contributes 5 and 4 electrons respectively. The whole charge of the molecule is -1 making the total number of valence electrons 10.
The 8 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on nitrogen atom to complete the octet.
Since the octets of carbon atoms are not filled, a triple bond is made between carbon and nitrogen atoms in expense of two electrons where the remaining four electrons are distributed over the atoms present in the given molecule.
(d)
Interpretation: The Lewis structures and the formal charges for the given isoelectronic species should be shown.
Concept Introduction:
- Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
- It is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
To find: The Lewis structure for the given set of isoelectronic species.
Answer to Problem 6.83QP

Explanation of Solution
Given isoelectronic species is below.
Lewis structure of above isoelectronic species is drawn below.

The total number of valence electrons is found to be 10, where both nitrogen atoms contribute 5 electrons.
The 8 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on nitrogen atom to complete the octet.
Since the octets of nitrogen atoms are not filled, a triple bond is made between both nitrogen atoms.
(a)
Interpretation: The formal charges for the given isoelectronic species should be shown.
Concept Introduction
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all chemical bonds are shared equally among atoms.
This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
The Lewis structure with formal charge on each of the atoms close to zero is taken as the most plausible structure.
Formal charge of an atom can be determined by the given formula.
Answer to Problem 6.83QP
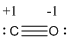
Explanation of Solution
Formal charge of the given isoelectronic species is given below
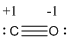
The formal charge of the given isoelectronic species is calculated,
- Carbon atom
Substituting these values to the equation,
- Oxygen atom
Substituting these values to the equation,
(b)
Interpretation: The formal charges for the given isoelectronic species should be shown.
Concept Introduction
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all chemical bonds are shared equally among atoms.
This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
The Lewis structure with formal charge on each of the atoms close to zero is taken as the most plausible structure.
Formal charge of an atom can be determined by the given formula.
Answer to Problem 6.83QP
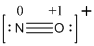
Explanation of Solution
Formal charge of the given isoelectronic species is given below
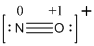
The formal charge of the given isoelectronic species is calculated,
- Nitrogen atom
Substituting these values to the equation,
- Oxygen atom
Substituting these values to the equation,
(c)
Interpretation: The formal charges for the given isoelectronic species should be shown.
Concept Introduction
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all chemical bonds are shared equally among atoms.
This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
The Lewis structure with formal charge on each of the atoms close to zero is taken as the most plausible structure.
Formal charge of an atom can be determined by the given formula.
Answer to Problem 6.83QP
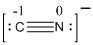
Explanation of Solution
Formal charge of the given isoelectronic species is given below
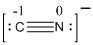
The formal charge of the given isoelectronic species is calculated,
- Carbon atom
Substituting these values to the equation,
- Nitrogen atom
Substituting these values to the equation,
(d)
Interpretation: The formal charges for the given isoelectronic species should be shown.
Concept Introduction
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all chemical bonds are shared equally among atoms.
This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
The Lewis structure with formal charge on each of the atoms close to zero is taken as the most plausible structure.
Formal charge of an atom can be determined by the given formula.
Answer to Problem 6.83QP
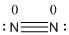
Explanation of Solution
Formal charge of the given isoelectronic species is given below
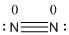
The formal charge of the given isoelectronic species is calculated,
- Nitrogen atom
Substituting these values to the equation,
- Since both the nitrogen atoms are similar, the formal charge of the nitrogen atoms is zero.
Want to see more full solutions like this?
Chapter 6 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- An ionic compound has the following composition (by mass): Ca, 30.3%; N, 21.2%; O, 48.5%. What are the formula and name of the compound? Write the Lewis formulas for the ions.arrow_forward7.107 How do the Lewis symbols for C, Si, and Ge reflect the similarity in their electron configurations?arrow_forwardThe ionic radii of element E and a different metallic element, M, are shown in the following table: Both elements form oxides, E2O and MO. If lattice energy is defined as the energy required to separate an ionic solid into individual separate gaseous ions, would the lattice energy of MO be less than, equal to, or greater than the lattice energy of the oxide E2O? Justify your answer in terms of Coulomb's lawarrow_forward
- Draw a Lewis electron-dot symbol for (a) Ba; (b) Kr; (c) Br.arrow_forwardThe cyanate ion, NCO– , has three (3) possible Lewis structures. (a) Draw these three structures and assign formal charges in each. (b) Which Lewis structure is dominant?arrow_forwardFor the Lewis electron dot formula for lanthanum sulfite, La2(SO3)3 , there are ___Regions of Electron Density around the S and the shape of the sulfite is ____arrow_forward
- Considering only ions with charges of +1, +2, -1 and -2, or neutral atoms, give the symbols for 4 species that are isoelectronic with the sulfide ion, S2-. Considering only ions with charges of +1, +2, -1 and -2, or neutral atoms, give the symbols for 4 species that are isoelectronic with Ar.arrow_forwardRank the following ionic compounds in order of lattice energy, from least exothermic to most exothermic: KCl, SrO, RbBr, CaOarrow_forwardDraw the lewis electron dot structures of the following atoms: Sb, Si, S, Se, Xearrow_forward
- Aluminum oxide (Al₂ O₃) is a widely used industrial abrasive(emery, corundum), for which the specific application depends onthe hardness of the crystal. What does this hardness imply about the magnitude of the lattice energy? Would you have predictedfrom the chemical formula that Al₂ O₃ is hard? Explain.arrow_forwardThe C¬O bond length in carbon monoxide, CO, is 1.13 Å, whereas the C¬Obond length in CO2 is 1.24 Å. Without drawing a Lewis structure, do you think thatCO contains a single, double, or triple bond?arrow_forwardwe would predict that the imaginary ionic compound XF would have a (higher /lower) lattice energy than YyF2 becausearrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning



