
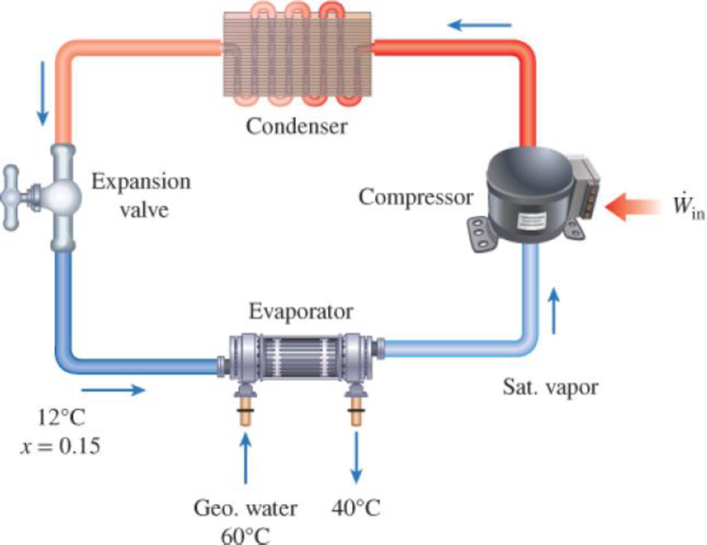
A heat pump with refrigerant-134a as the working fluid is used to keep a space at 25°C by absorbing heat from geothermal water that enters the evaporator at 60°C at a rate of 0.065 kg/s and leaves at 40°C. Refrigerant enters the evaporator at 12°C with a quality of 15 percent and leaves at the same pressure as saturated vapor. If the compressor consumes 1.6 kW of power, determine (a) the mass flow rate of the refrigerant, (b) the rate of heat supply, (c) the COP, and (d) the minimum power input to the compressor for the same rate of heat supply.
FIGURE P6–152
(a)
The mass flow rate of the refrigerant.
Answer to Problem 152RP
The mass flow rate of the refrigerant is
Explanation of Solution
Determine the rate of heat absorbed from the water.
Here, the mass flow rate of the water is
Determine the mass flow rate of a refrigerant.
Conclusion:
From the Table A-11, “Saturated refrigerant R-134a”, obtain the value of saturated pressure of the refrigerant at the inlet temperature of
Here, the pressure of refrigerant is constant in evaporation.
From the Table A-11, “Saturated refrigerant R-134a” to obtain the value of specific enthalpy of the refrigerant at the outlet pressure of
From the Table A-11, “Saturated refrigerant R-134a” to obtain the value of specific enthalpy of saturated liquid and specific enthalpy change upon vaporization of the refrigerant at the inlet temperature of
Calculate the specific enthalpy of refrigerant at evaporator inlet.
Here, the specific enthalpy of saturated liquid is
Substitute
From the Table A-4, “Saturated water-temperature” to obtain the value of specific enthalpy of saturated liquid of water at the inlet temperature of
From the Table A-4, “Saturated water-temperature” to obtain the value of specific enthalpy of saturated liquid of water at the outlet temperature of
Substitute
Substitute
Thus, the mass flow rate of the refrigerant is
(b)
The heating load of the heat pump.
Answer to Problem 152RP
The heating load of the heat pump is
Explanation of Solution
Determine the heating load of the heat pump.
Here, the power input consumed by compressor is
Conclusion:
Substitute
Thus, the heating load of the heat pump is
(c)
The COP of a heat pump operating between the same temperature limits.
Answer to Problem 152RP
The COP of a heat pump operating between the same temperature limits is
Explanation of Solution
Determine the coefficient of performance of the heat pump.
Conclusion:
Substitute
Thus, the COP of a heat pump operating between the same temperature limits is
(d)
The minimum power input to the compressor.
Answer to Problem 152RP
The minimum power input to the compressor is
Explanation of Solution
Determine the maximum coefficient of performance of the heat pump operating between the same temperature limits.
Here, the temperature of higher temperature body is
Determine the minimum power input to the condenser for the same heat pump load.
Conclusion:
Substitute
Substitute
Thus, the minimum power input to the compressor is
Want to see more full solutions like this?
Chapter 6 Solutions
Thermodynamics: An Engineering Approach
- A heat pump with refrigerant-134a as the working fluid is used to keep a space at 25°C by absorbing heat from geothermal water that enters the evaporator at 60°C at a rate of 0.065 kg/s and leaves at 40°C. Refrigerant enters the evaporator at 12°C with a quality of 15 percent and leaves at the same pressure as saturated vapor. If the compressor consumes 1.6 kW of power, determine the COP.arrow_forwardRefrigerant-134a at a rate of 0.08 kg/s enters the compressor of a refrigerator as superheated vapor at 0.18 MPa and 0 ℃ and leaves at 0.9 MPa and 80 ℃. The refrigerant is cooled in the condenser to 31.3 ℃ and 0.8 MPa and it is throttled to 0.18 MPa. Disregarding any heat transfer and pressure drops in the connecting lines between the components, Determine the adiabatic efficiency of the compressorarrow_forwardA steam power plant receives heat from a furnace at a rate of 280 GJ/h. Heat losses to the surrounding air from the steam as it passes through the pipes and other components are estimated to be about 8 GJ/h. If the waste heat is transferred to the cooling water at a rate of 165 GJ/h, determine the thermal efficiency of this power plant.arrow_forward
- An air conditioner using refrigerant-134a as the working fluid is used to keep the temperature of a room at 23°C by giving heat to the external environment at 37°C. The heat gain of the house from the walls and windows is 250 kJ/min; 900 W heat is emitted into the room from the computer, TV and lamps. The refrigerant enters the compressor with a flow rate of 100 L/min in the form of saturated vapor at 400 kPa pressure and leaves the compressor at 70°C at 1200 kPa pressure.a) Draw the cycle by showing the elements of the cycle.b) the actual COP value,c) The highest COP value,d) The smallest refrigerant can have for the same compressor inlet and outlet conditions.Calculate the volumetric flow.arrow_forwardAn air-conditioner with refrigerant-134a as the working fluid is used to keep a room at 30°C by rejecting the waste heat to the outdoor air at 50°C. The room gains heat through the walls and the windows at a rate of 350 kJ/min while the heat generated by the computer, TV, and lights amounts to 1000 W. The refrigerant enters the compressor at 400 kPa as a saturated vapor at a rate of 120 L/min and leaves at 1400 kPa and 70°C. Determine: (a) the actual COP, (b) the maximum COP, and (c) the minimum volume flow rate of the refrigerant at the compressor inlet for the same compressor inlet and exit conditions.arrow_forwardConsider a Carnot heat-engine cycle executed in a steady-flow system using steam as the working fluid. The cycle has a thermal efficiency of 30 percent, and steam changes from saturated liquid to saturated vapor at 275°C during the heat addition process. If the mass flow rate of the steam is 3 kg/s, determine the net power output of this engine, in kW.arrow_forward
- Refrigerant-134a enters the condenser of a residential heat pump at 800 kPa and 35°C at a rate of 0.018 kg/s and leaves at 800 kPa as a saturated liquid. If the compressor consumes 1.2 kW of power, determine the rate of heat absorption from the outside air.arrow_forwardA commercial refrigerator with refrigerant-134a as the working fluid is used to keep the refrigerated space at –35°C by rejecting waste heat to cooling water that enters the condenser at 18°C at a rate of 0.25 kg/s and leaves at 26°C. The refrigerant enters the condenser at 1.2 MPa and 50°C and leaves at the same pressure subcooled by 5°C. If the compressor consumes 3.3 kW of power, determine the mass flow rate of the refrigerant,arrow_forwardA commercial refrigerator with refrigerant-134a as the working fluid is used to keep the refrigerated space at –35°C by rejecting waste heat to cooling water that enters the condenser at 18°C at a rate of 0.25 kg/s and leaves at 26°C. The refrigerant enters the condenser at 1.2 MPa and 50°C and leaves at the same pressure subcooled by 5°C. If the compressor consumes 3.3 kW of power, determine the refrigeration load.arrow_forward
- A commercial refrigerator with refrigerant-134a as the working fluid is used to keep the refrigerated space at –35°C by rejecting waste heat to cooling water that enters the condenser at 18°C at a rate of 0.25 kg/s and leaves at 26°C. The refrigerant enters the condenser at 1.2 MPa and 50°C and leaves at the same pressure subcooled by 5°C. If the compressor consumes 3.3 kW of power, determine the COP,arrow_forwardA commercial refrigerator with refrigerant-134a as the working fluid is used to keep the refrigerated space at –35°C by rejecting waste heat to cooling water that enters the condenser at 18°C at a rate of 0.25 kg/s and leaves at 26°C. The refrigerant enters the condenser at 1.2 MPa and 50°C and leaves at the same pressure subcooled by 5°C. If the compressor consumes 3.3 kW of power, determine the minimum power input to the compressor for the same refrigeration load.arrow_forwardRefrigerant-134a enters the compressor of a refrigerator at 0.14MPa and -10°C at a rate of 0.05 kg/s and leaves at 0.8 MPa and 50°C. The refrigerant is cooled in the condenser to 26°C and 0.72 MPa and is throttled to 0.15 MPa. Determine (a) the rate of heat removal from the refrigerated space and the power input to the compressor, (b) the isentropic efficiency of the compressor, and (c) the coefficient of performance of the refrigerator. Answers: (a) 7.93 kW, 2.02 kW, (b) 0.939, (c) 3.93arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





