
a)
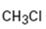
Interpretation:
Whether CH3Cl is likely to behave as a nucleophile or electrophile or both to be stated.
Concept introduction:
A nucleophile (either negatively charged or a neutral molecule containing an atom with at least one lone pair of electrons) has an electron rich-site. An electrophile (either positively charged or a neutral molecule containing an electron deficient atom) has an electron poor-site.
To state:
Whether CH3Cl will behave as nucleophile or electrophile or both.
b)
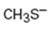
Interpretation:
Whether CH3S- is likely to behave as a nucleophile or electrophile or both to be stated.
Concept introduction:
A nucleophile (either negatively charged or a neutral molecule containing an atom with at least one lone pair of electrons) has an electron rich-site. An electrophile (either positively charged or a neutral molecule containing an electron deficient atom) has an electron poor-site.
To state:
Whether CH3S- will behave as nucleophile or electrophile or both.
c)
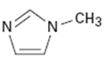
Interpretation:
Whether the compound given is likely to behave as a nucleophile or electrophile or both to be stated.
Concept introduction:
A nucleophile (either negatively charged or a neutral molecule containing an atom with at least one lone pair of electrons) has an electron rich-site. An electrophile (either positively charged or a neutral molecule containing an electron deficient atom) has an electron poor-site.
To state:
Whether the compound given will behave as nucleophile or electrophile or both.
d)
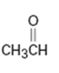
Interpretation:
Whether CH3CHO is likely to behave as a nucleophile or electrophile or both to be stated.
Concept introduction:
A nucleophile (either negatively charged or a neutral molecule containing an atom with at least one lone pair of electrons) has an electron rich-site. An electrophile (either positively charged or a neutral molecule containing an electron deficient atom) has an electron poor-site.
To state:
Whether CH3CHO will behave as nucleophile or electrophile or both.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry
- Which of the following species cannot be considered an electrophile?arrow_forwardIdentify the likely electrophilic and nucleophilic sites in each of the following molecules:arrow_forwardWhat are the list of difference between SN1, SN2, E1, and E2? (such as the type of nucleophiles needed, the type of substrates, solvents etc.)arrow_forward
- Decide which compounds from the list below are best suited for nucleophilic addition reactions and which ones are more appropriate for nucleophilic substitution reactions.arrow_forwardsee the attached question and out of these 3 comounds, which one is the best dienophile ?arrow_forwardWhat are the organic products of these reactions? Are they electrophiles or nucleophiles? What is their decreasing order of rate?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
