
7-11 Consider the following reaction:
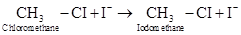
Suppose we start the reaction with an initial iodomethane concentration of 0.260 M. This concentration increases to 0.840 M over a period of 1 h 20 min. What is the
Interpretation:
The rate of given reaction with initial concentration of iodomethane0.26 m and increased to 0.84 m in 1 h 20 min should be determined.
Concept Introduction:
Rate of reaction:
The rate of any reaction depends on the concentration change of limiting reagent formed in a reaction.
Concentration:
Concentration of all chemical species takes part in a reaction depend on their moles present in per liter solution.
Answer to Problem 1P
Rate of reaction = 0.00725 M/min.
Explanation of Solution
Given information:
Reaction with initial concentration of iodomethane 0.26 m that increased to 0.84 m in 1 h 20 min.
Put the given values in above expression.
Rate of reaction = 0.00725 M/min.
Want to see more full solutions like this?
Chapter 7 Solutions
Introduction To General, Organic, And Biochemistry
- 7-35 A reaction has a high rate constant but a small equilibrium constant. What does this mean in terms of producing an industrial product?arrow_forward7-13 Why are reactions between ions in aqueous solution generally much faster than reactions between covalent molecules?arrow_forward7-10 The rate of disappearance of HCI was measured for the following reaction: The initial concentration of HCI is 1.85 M. Its concentration decreases to 1.58 M in 54.0 min. What is the rate of reaction?arrow_forward
- . Account for the increase in reaction rate brought about by a catalyst.arrow_forward7-50 Draw an energy diagram for an exothermic reaction that yields 75 kcal/mol. The activation energy is 30 kcal/mol.arrow_forwardIn general, can we predict the effect of doubling the concentration of A on the rate of the overall reaction A+BC? Can we predict the effect if the reaction is known to be an elementary reaction?arrow_forward
- How do chemists envision reactions taking place in terms of the collision model for reactions? Give an example of a simple reaction and how you might envision the reaction’s taking place by means of a collision between the molecules.arrow_forwardGo to the PhET Reactions and change to Angled shot to see the difference. (a) What happens when the angle of the collision is changed? (b) Explain how this is relevant to rate of reaction.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





