
Concept explainers
Interpretation:
The diagrams of waves are to be drawn, the wavelength that X-rays and radio waves have on this scale is to be calculated and the range of wavelength visible to our eyes are to be determined.
Concept introduction:
The distance between two consecutive crests of a wave of light is called the
The arrangement of lights according to the wavelength associated with it, is called a spectrum.
To convert the m to nm, the conversion factor is as:
To convert nm to mm, the conversion factor is as:
Answer to Problem 36E
Solution: The diagram of a wave of the violet edge
The wavelength that X-rays and radio waves have on this scale are
Explanation of Solution
Given information:
The violet edge of spectrum appears at a wavelength of
The wavelength of X-rays, radio waves and visible range in mm is calculated as follows:
For X-rays,
For radio waves,
For violet and red edged visible range,
For Violet edge:
For Red edge:
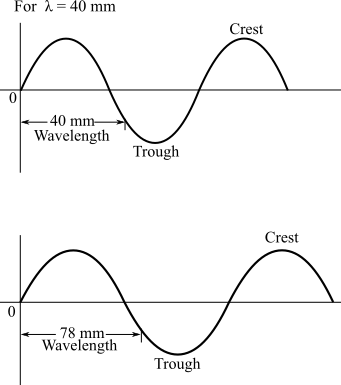
The sketches of the waves associated with wave lengths 40mm and 78 mm are as:

Diagrams of waves are drawn as follows;

The X-rays and radio waves on this scale are
Want to see more full solutions like this?
Chapter 7 Solutions
Chemistry In Focus
- An FM radio station found at 103.1 on the FM dial broadcasts at a frequency of 1.031188s1 (103.1 MHz). What is the wavelength of these radio waves in meters?arrow_forwardTraffic signals are often now made of LEDs (light-emitting diodes). Amber and green ones are pictured here. (a) The light from an amber signal has a wave-length of 595 nm, and that from a green signal has a wavelength of 500 nm. Which has the higher frequency? (b) Calculate the frequency of amber light.arrow_forwardA baseball weighs 142 g. A professional pitcher throws a fast ball at a speed of 100 mph and a curve ball at 80 mph. What wavelengths are associated with the motions of the baseball? If the uncertainty in the position of the ball is 12 wavelength, which ball (fast ball or curve) has a more precisely known position? Can the uncertainty in the position of a curve ball be used to explain why batters frequently miss it?arrow_forward
- You are an engineer designing a switch that works by the photoelectric effect. The metal you wish to use in your device requires 6.7 1019 J/atom to remove an electron. Will the switch work if the light falling on the metal has a wavelength of 540 nm or greater? Why or why not?arrow_forward(a) Which color in the visible spectrum has the highest frequency? Which has the lowest frequency? (b) Is the wavelength of the radiation used in a microwave oven (2.45 GHz) longer or shorter than that from your favorite FM radio station (for example, 91.7 MHz)? (c) Are the wavelengths of x-rays longer or shorter than those of ultraviolet light? (d) Calculate the frequency of green light with a wavelength of 510. nm.arrow_forwardThe lasers used in supermarket scanners emit red light at a wavelength of 633 nm. Compact disc players use lasers that emit light (that is not visible) at 840 nm. Which photonsthose emitted by supermarket scanners or compact disc (CD) playerscontain more energy per photon? Supermarket scanners CD players They both contain the same amount of energy per photon.arrow_forward
- This laser emits green light with a wavelength of 533 nm. (a) What is the energy, in joules, of one photon of light at this wavelength? (b) If a particular laser produces 1.00 watt (W) of power (1 W = 1 J/s), how many photons are produced each second by the laser?arrow_forwardRGB color television and computer displays use cathode ray tubes that produce colors by mixing red, green, and blue light. If we look at the screen with a magnifying glass, we can see individual dots turn on and off as the colors change. Using a spectrum of visible light, determine the approximate wavelength of each of these colors. What is the frequency and energy of a photon of each of these colors?arrow_forward
 Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





