
Concept explainers
(a)
Interpretation: The name of the given compound has to be stated.
Concept introduction: In a cyclic monosaccharide, the replacement of an
(a)
Answer to Problem 7.110EP
The name of the given structure is ethyl-
Explanation of Solution
The Fisher projection formula for D-allose is,
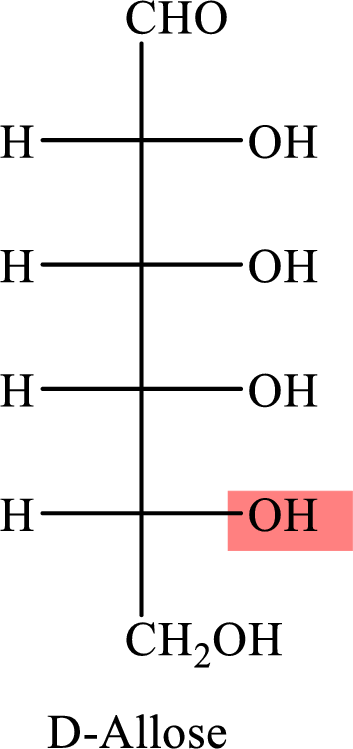
The structure given in Problem 18-104 is,
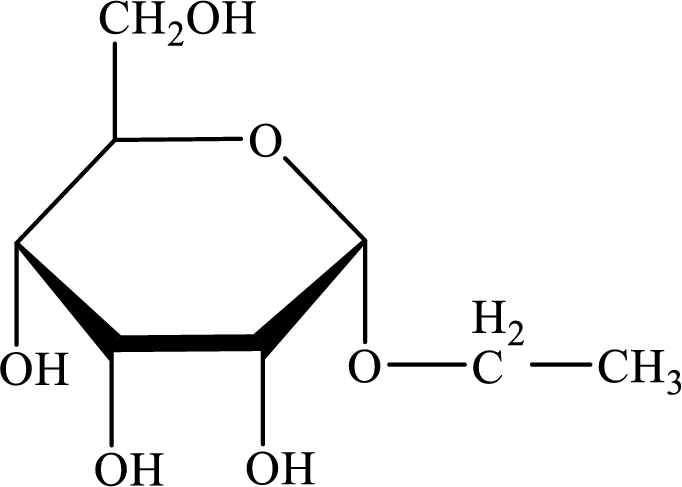
The above structure is the cyclic form of monosaccharide D-allose. Both the
(b)
Interpretation: The name of the given compound has to be stated.
Concept introduction: In a cyclic monosaccharide, the replacement of an
(b)
Answer to Problem 7.110EP
The name of the given structure is methyl-
Explanation of Solution
The Fisher projection formula for D-allose is,
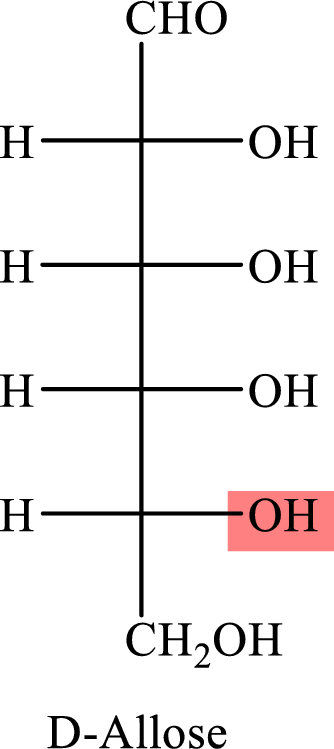
The structure given in Problem 18-104 is,
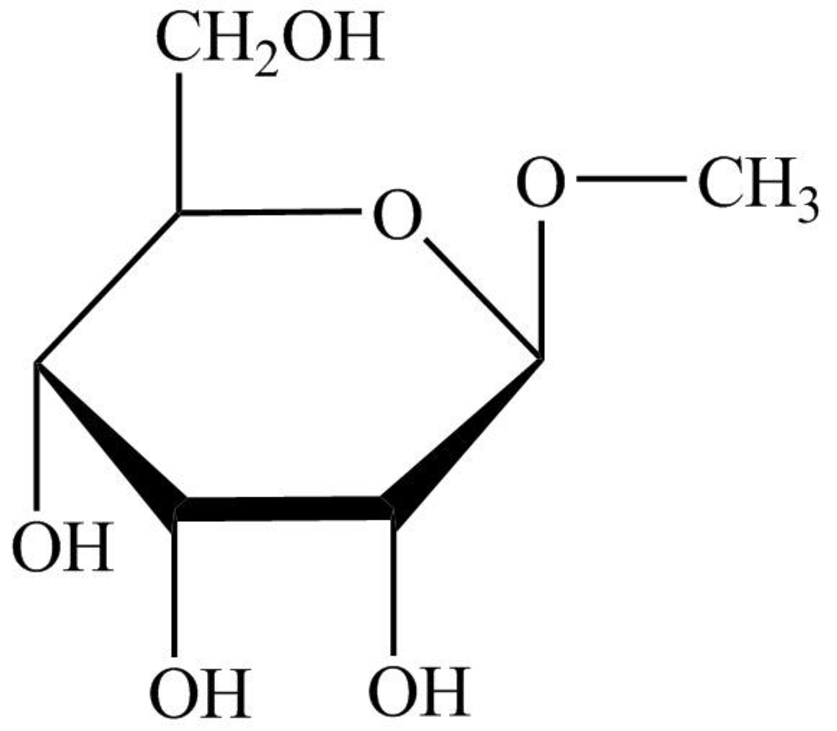
The above structure is the cyclic form of monosaccharide D-allose. Both the
(c)
Interpretation: The name of the given compound has to be stated.
Concept introduction: In a cyclic monosaccharide, the replacement of an
(c)
Answer to Problem 7.110EP
The name of the given structure is ethyl-
Explanation of Solution
The Fisher projection formula for D-psicose is,
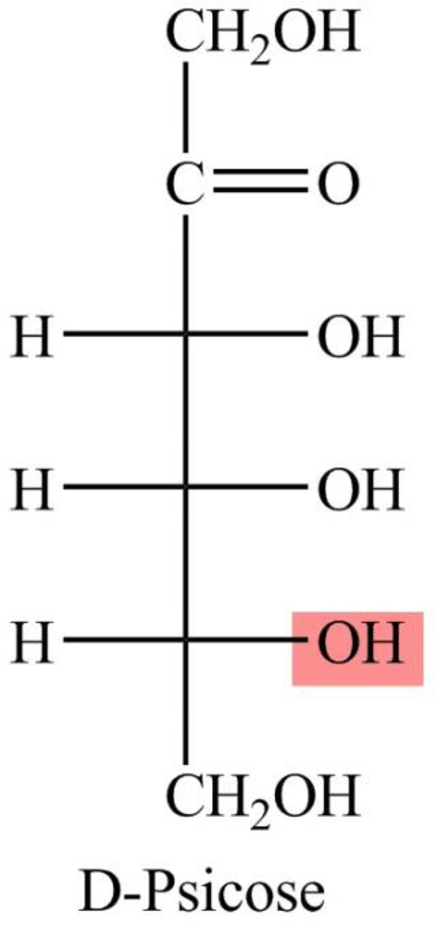
The structure given in Problem 18-104 is,
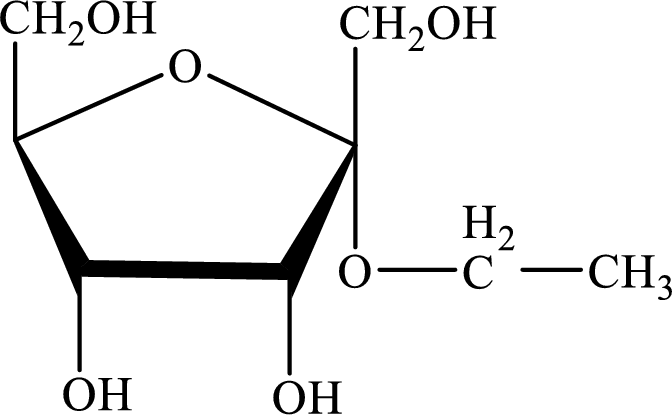
The above structure is the cyclic form of monosaccharide D-psicose. Both the
(d)
Interpretation: The name of the given compound has to be stated.
Concept introduction: In a cyclic monosaccharide, the replacement of an
(d)
Answer to Problem 7.110EP
The name of the given structure is methyl-
Explanation of Solution
The Fisher projection formula for D-mannose is,
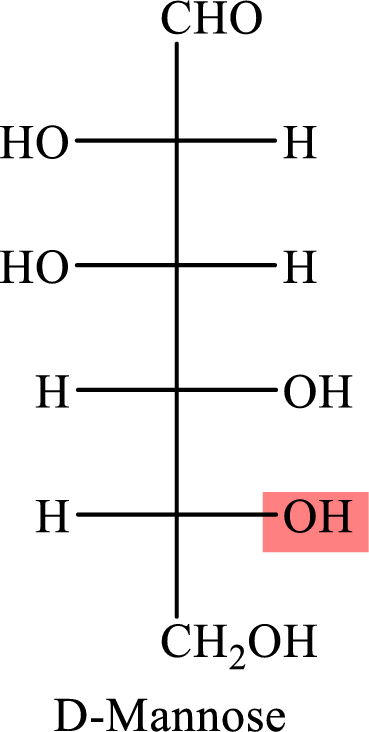
The structure given in Problem 18-104 is,
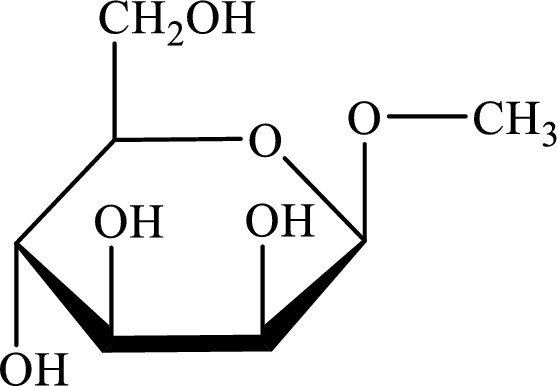
The above structure is the cyclic form of monosaccharide D-mannose. Both the
Want to see more full solutions like this?
Chapter 7 Solutions
Organic And Biological Chemistry
- Using the information given in Table 19-1, assign an IUPAC name to each of the following fatty acids. a. Stearic acid b. Linolenic acidarrow_forwardFor each of the triacylglycerol molecules in Problem 19-36, indicate how many of each of the following entities are present in the molecule. In certain cases, the answer may be zero. a. Omega-3 fatty acid residues b. Omega-6 fatty acid residues c. Bad fatty acid residues d. 9,12 fatty acid residuesarrow_forward21-48 List all of the functional groups that make taurocholate water-soluble.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning



