
Concept explainers
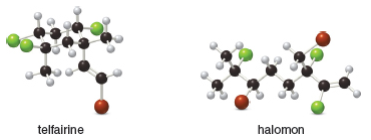
Telfairine, a naturally occurring insecticide, and halomon, an antitumor agent, are two
polyhalogenated compounds isolated from red algae. (a) Classify each halide bonded to an
hybridized carbon as 1°, 2°, or 3°. (b) Label each halide as vinyl, allylic, or neither.
(a)
Interpretation: The halide bonded to
Concept introduction: A halide is classified as
Answer to Problem 7.1P
The halide bonded to
Explanation of Solution
The ball and stick model of telfarine and halomon shows that black balls represent carbon atom, white balls represent hydrogen atom, green and brown balls represent chlorine and bromine atom, respectively.
The actual structure of telfarine and halomon is shown in Figure 1.
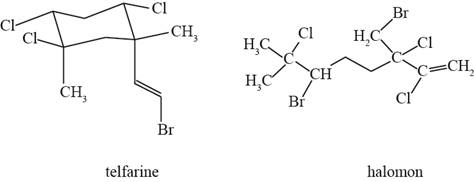
Figure 1
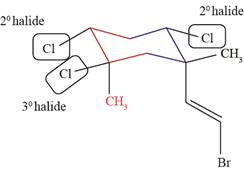
A halide is classified as
The
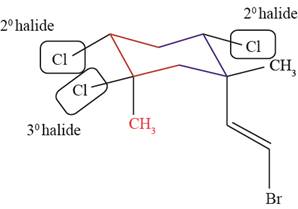
Figure 2
The
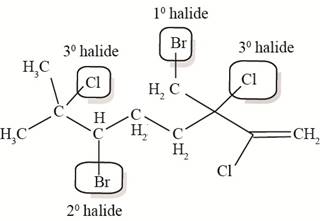
Figure 3
The halide bonded to
(b)
Interpretation: Each halide is to be labeled as vinyl, allylic or neither.
Concept introduction: In allyl halide, halogen atom is bonded to
Answer to Problem 7.1P
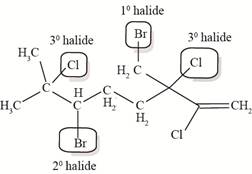
The allylic and vinyl halides of telfarine and halomon are shown below.
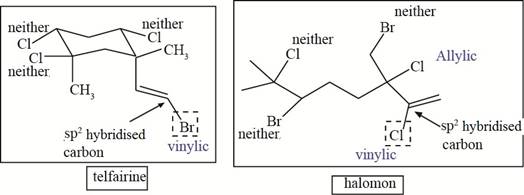
Explanation of Solution
In allyl halide, halogen atom is bonded to
The allylic and vinyl halides of telfarine and halomon are shown in Figure 4.
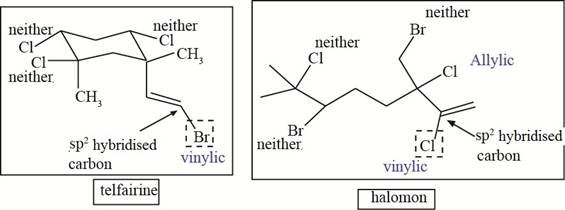
Figure 4
Each halide is labeled as allylic, vinyl and neither as shown in Figure 4.
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Essential Organic Chemistry (3rd Edition)
General, Organic, and Biological Chemistry - 4th edition
Inorganic Chemistry
EBK INTRODUCTION TO CHEMISTRY
General Chemistry: Atoms First
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
- Under certain reaction conditions, 2,3-dibromobutane reacts with twoequivalents of base to give three products, each of which contains twonew π bonds. Product A has two sp hybridized carbon atoms, product Bhas one sp hybridized carbon atom, and product C has none. What arethe structures of A, B, and C?arrow_forwardDraw the structure of each alkene of molecular formula C7H14 that has a tetrasubstituted double bondarrow_forwardA is a toxin produced by the poisonous seaweed Chlorodesmis fastigiata. (a) Label each alkene that exhibits stereoisomerism as E or Z. (b) Draw a stereoisomer of A that has all Z double bonds.arrow_forward
- Draw the organic product(s) formed upon the addition of HBr to (a) 2-methyl-2-pentene, (b) trans-2-hexene, and (c) 4-methylcyclohexene. How many regioisomers can be formed in each case?arrow_forwardThe shrub ma huang (Section 5.4A) contains two biologically activestereoisomers—ephedrine and pseudoephedrine—with two stereogeniccenters as shown in the given structure. Ephedrine is one component ofa once-popular combination drug used by body builders to increaseenergy and alertness, whereas pseudoephedrine is a nasaldecongestant.a.) Draw the structure of naturally occurring (−)-ephedrine, which has the1R,2S configuration.b.) Draw the structure of naturally occurring (+)-pseudoephedrine, whichhas the 1S,2S configuration.c.) How are ephedrine and pseudoephedrine related?d.) Draw all other stereoisomers of (−)-ephedrine and (+) pseudoephedrine, and give the R,S designation for all stereogeniccenters.e.) How is each compound drawn in part (d) related to (−)-ephedrine?arrow_forwardDraw the constitutional isomer formed when the attached alkenes are treated with each set of reagents: [1] H2O, H2SO4; or [2] BH3 followed by H2O2, −OH.arrow_forward
- Draw the organic products formed when cyclopentene is treated withfollowing reagent. CH3CO3Harrow_forwardWhat product is formed when benzene is treated with each organic halide in the presence of AlCl3?arrow_forwardCan an aldehyde have molecular formula C 5H 12O? Explain why or why not.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





