
Concept explainers
Consider the following

a. Draw a mechanism using curved arrows.
b. Draw an energy diagram. Label the axes, the reactants, products,
c. Draw the structure of the transition state.
d. What is the rate equation?
e. What happens to the reaction rate in each of the following instances? [1] The leaving group is changed from
(a)
Interpretation: The mechanism of the given reaction is to be drawn by the use of curved arrows.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons are known as a nucleophile. In a nucleophilic substitution reaction, nucleophile takes the position of leaving group by attacking the electron deficient carbon atom.
Answer to Problem 7.53P
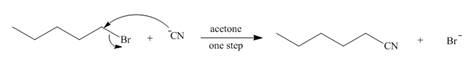
The mechanism of the given reaction is,
Explanation of Solution
The structure of the given alkyl halide shows that carbon atom, on which bromine is present, is bonded to one another carbon atom. Hence, the bromine atom is bonded to primary carbon atom and the given alkyl halide is
In

Figure 1
The mechanism of the given reaction is shown in Figure 1.
(b)
Interpretation: The energy diagram is to be drawn. The axes, reactants, products,
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The graphical representation of chemical reaction in which x-axis represents energy of the reaction and y-axis represents the reaction coordinate is called energy profile diagram.
Answer to Problem 7.53P
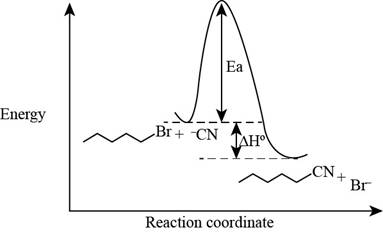
The energy diagram of the given reaction is shown below.
Explanation of Solution
In bimolecular nucleophilic substitution reaction, formation and breakage of bond takes place simultaneously in the transition state. In the given reaction, breakage of
The energy of products is lower than the energy of reactants in exothermic reaction. Therefore, the energy diagram of the given reaction is,
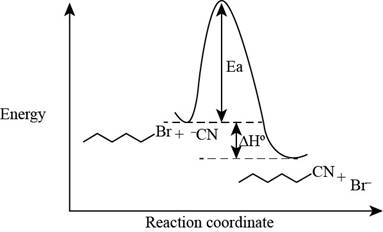
Figure 2
The x-axes of the energy diagram represent the energy of reactants and products and y-axes represent the reaction coordinate.
The energy diagram of the given reaction is shown in Figure 2.
(c)
Interpretation: The structure of the transition state is to be drawn.
Concept introduction: In bimolecular nucleophilic substitution reaction, formation and breakage of bond takes place simultaneously in the transition state. The energy of transition state is more than the energy of reactants and products.
Answer to Problem 7.53P
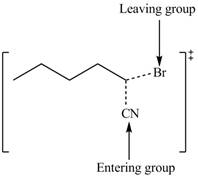
The structure of the transition state is shown below.
Explanation of Solution
In bimolecular nucleophilic substitution reaction, formation and breakage of bond takes place simultaneously in the transition state. In the given reaction, breakage of
Therefore, the transition state of the given reaction is,
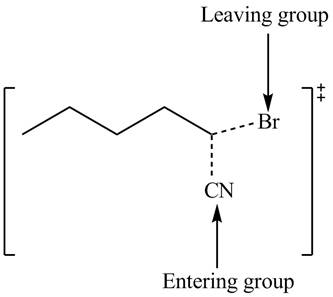
Figure 3
The structure of the transition state is shown in Figure 3.
(d)
Interpretation: The rate equation of the given reaction is to be predicted.
Concept introduction: The rate of
The rate equation of
Answer to Problem 7.53P
The rate equation of the given reaction is,
Explanation of Solution
The rate of
The rate equation of
The alkyl halide of given reaction is
Therefore, the rate equation of given reaction is,
The rate equation of the given reaction is,
(e)
Interpretation: The change that occurs to the reaction rate in given instances is to be stated.
Concept introduction: The rate of
The rate of
Answer to Problem 7.53P
The change that occurs to the reaction rate in given instances is,
[1] The rate of the reaction will increase.
[2] The rate of the reaction will decrease.
[3] The rate of the reaction will decrease.
[4] The rate of the reaction increases by five times.
[5] The rate of the reaction increases by twenty five times.
Explanation of Solution
[1]
The primary halide undergoes nucleophilic substitution by
[2]
The polar protic solvent favors
[3]
The primary halide undergoes nucleophilic substitution by
[4]
The rate of
The rate of
According the given statement, the concentration of
Therefore, the rate of the reaction increases by five times when the concentration of
[5]
The rate of
The rate of
According the given statement, the concentration of
Therefore, the rate of the reaction increases by twenty five times when the concentration
The change that occurs to the reaction rate in given instances is,
[1] The rate of the reaction will increase.
[2] The rate of the reaction will decrease.
[3] The rate of the reaction will decrease.
[4] The rate of the reaction increases by five times.
[5] The rate of the reaction increases by twenty five times.
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry
Chemistry
Introduction to Chemistry
Elementary Principles of Chemical Processes, Binder Ready Version
General Chemistry: Principles and Modern Applications (11th Edition)
- 1. For the following reaction, what is the rate law ? (image) 2. is the following nucleophile strong or weak HO^- a) strong b) weak c) not a nucleophile 3) what set of reaction conditions should favor an SN2 reaction on 2-bromo-3-methylbutane a) weak nucleophile in a protic solvent b) weal nucleophile in aprotic solvent c) strong nucleophile in a protic solvent d) strong nucleophile in a aprotic solventarrow_forward1. What type of reaction is occuring in step 3? (halogenation, hydrohalogenation, reduction, keto–enol tautomerism, dehydrohalogenation, acid-catalyzed hydration, base-catalyzed hydration) 2. Which reagent is necessary for step 3? (Br2, HBr, H2/Pt, NaNH2, H20/H2SO4/HgSO4)arrow_forwardConsider the following SN2 reaction.a.Draw a mechanism using curved arrows. b. Draw an energy diagram. Label the axes, the reactants, products, Ea, and ΔH°. Assume that the reaction is exothermic. c. Draw the structure of the transition state. d.What is the rate equation? e.What happens to the reaction rate in each of the following instances? [1] The leaving group is changed from Br− to I−; [2] The solvent is changed from acetone to CH3CH2OH; [3] The alkyl halide is changed from CH3(CH2)4Br to CH3CH2CH2CH(Br)CH3; [4] The concentration of −CN is increased by a factor of five; and [5] The concentrations of both the alkyl halide and −CN are increased by a factor of five.arrow_forward
- What kind of reagent is KmnO4? Draw the possible reactions of this reagent with the specific compounds tested in the following reaction?arrow_forwardDraw the rearrangement mechanism for the following image #10arrow_forwardWhy is the mechanism shown in Figure 28 not plausible? * A- The reagent does not have a good leaving group. B- Water is too weak a base for the reaction to occur. C- The structure of the haloalkane prevents the nucleophile from coming close to the alpha carbon atom. D- None of these statements are correct. E- All these statements are correct.arrow_forward
- Consider the attached E2 reaction What happens to the reaction rate with each of the following changes?[1] The solvent is changed to DMF. [2] The concentration of −OC(CH3)3 is decreased. [3] The base is changed to −OH. [4] The halide is changed to CH3CH2CH2CH2CH(Br)CH3. [5] The leaving group is changed to I−.arrow_forward7. Choose the best reagent from the list below for carrying out each transformation. Place the letter of the reagent in the blank to the left of the reaction. (see attached screenshot). a. 1. O3 2. Zn, H3O+ b. 1. BH3, THF 2. H2O2, NaOH, H2O c. H2O, H2SO4, heat d. 1. OsO4 2. NaHSO3, H2O e. KMnO4, acid f. 1. Hg(OAc)2, H2O 2. NaBH4arrow_forwardThe use of curved arrows is a powerful tool that illustrates even complex reactions. a.Add curved arrows to show how carbocation A is converted to carbocation B. Label each new σ bond formed. Similar reactions have been used in elegant syntheses of steroids. b.Draw the product by following the curved arrows. This reaction is an example of a [3,3] sigmatropic rearrangement, as we will learn in Chapter 25.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


