
Concept explainers
Which of the following pairs of atomic orbitals of adjacent nuclei can overlap to form a sigma bond? Which overlap to form a pi bond? Which cannot overlap (no bond)? Consider the x axis to be the internuclear axis, that is, the line joining the nuclei of the two atoms, (a) 1s and 1s, (b) 1s and 2px. (c) 2px and 2py, (d) 3py and 3py, (e) 2px and 2px. (f) 1s and 2s.
Interpretation:
From the given set of orbitals, the ones that forms sigma bond, the ones that forms pi bond and the ones that doesn’t form any bond have to be identified.
Concept Introduction:
There are two different types of chemical bond – sigma bond and pi bond. A bond between two atoms is known as sigma bond if the atomic orbitals of the atoms overlap end to end – it is also called head on overlapping. A bond is said to pi bond if it is formed by sideways overlapping of atomic orbitals of the atoms. This is also known as lateral overlapping.
Answer to Problem 7.61QP
Solution:
| S.No |
Set of atomic orbitals | Type of bond formation |
| (a) |
| Sigma bond |
Explanation of Solution
Two
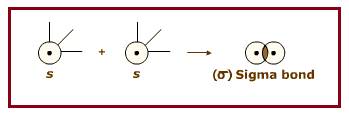
End to end overlapping of two
Figure 1
For example, the sigma bond between two Hydrogen atoms formed by end to end overlapping of
Interpretation:
From the given set of orbitals, the ones that forms sigma bond, the ones that forms pi bond and the ones that doesn’t form any bond have to be identified.
Concept Introduction:
There are two different types of chemical bond – sigma bond and pi bond. A bond between two atoms is known as sigma bond if the atomic orbitals of the atoms overlap end to end – it is also called head on overlapping. A bond is said to pi bond if it is formed by sideways overlapping of atomic orbitals of the atoms. This is also known as lateral overlapping.
Answer to Problem 7.61QP
Solution:
| S.No |
Set of atomic orbitals | Type of bond formation |
| (b) |
| Sigma bond |
Explanation of Solution
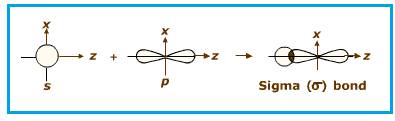
End to end overlapping of
Figure 2
Interpretation:
From the given set of orbitals, the ones that forms sigma bond, the ones that forms pi bond and the ones that doesn’t form any bond have to be identified.
Concept Introduction:
There are two different types of chemical bond – sigma bond and pi bond. A bond between two atoms is known as sigma bond if the atomic orbitals of the atoms overlap end to end – it is also called head on overlapping. A bond is said to pi bond if it is formed by sideways overlapping of atomic orbitals of the atoms. This is also known as lateral overlapping.
Answer to Problem 7.61QP
Solution:
| S.No |
Set of atomic orbitals | Type of bond formation |
| (c) |
| No bond formation |
Explanation of Solution
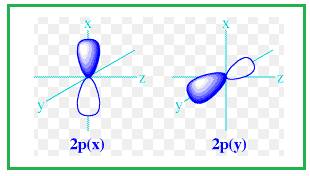
Shape of
Figure 3
Interpretation:
From the given set of orbitals, the ones that forms sigma bond, the ones that forms pi bond and the ones that doesn’t form any bond have to be identified.
Concept Introduction:
There are two different types of chemical bond – sigma bond and pi bond. A bond between two atoms is known as sigma bond if the atomic orbitals of the atoms overlap end to end – it is also called head on overlapping. A bond is said to pi bond if it is formed by sideways overlapping of atomic orbitals of the atoms. This is also known as lateral overlapping.
Answer to Problem 7.61QP
Solution:
| S.No |
Set of atomic orbitals | Type of bond formation |
| (d) |
| Pi bond |
Explanation of Solution
The
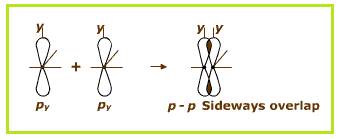
Sideways overlapping of two
Figure 4
Interpretation:
From the given set of orbitals, the ones that forms sigma bond, the ones that forms pi bond and the ones that doesn’t form any bond have to be identified.
Concept Introduction:
There are two different types of chemical bond – sigma bond and pi bond. A bond between two atoms is known as sigma bond if the atomic orbitals of the atoms overlap end to end – it is also called head on overlapping. A bond is said to pi bond if it is formed by sideways overlapping of atomic orbitals of the atoms. This is also known as lateral overlapping.
Answer to Problem 7.61QP
Solution:
| S.No |
Set of atomic orbitals | Type of bond formation |
| (e) |
| Pi bond |
Explanation of Solution
The
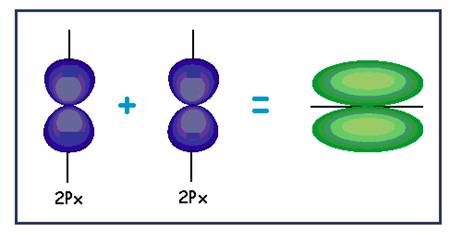
Lateral overlapping of two
Figure 5
Interpretation:
From the given set of orbitals, the ones that forms sigma bond, the ones that forms pi bond and the ones that doesn’t form any bond have to be identified.
Concept Introduction:
There are two different types of chemical bond – sigma bond and pi bond. A bond between two atoms is known as sigma bond if the atomic orbitals of the atoms overlap end to end – it is also called head on overlapping. A bond is said to pi bond if it is formed by sideways overlapping of atomic orbitals of the atoms. This is also known as lateral overlapping.
Answer to Problem 7.61QP
Solution:
| S.No |
Set of atomic orbitals | Type of bond formation |
| (f) |
| Sigma bond |
Explanation of Solution
Overlapping of two s-orbitals always result in sigma bond formation as two s-orbitals overlap head to head as follows –

End to end overlapping of
Figure 6
Want to see more full solutions like this?
Chapter 7 Solutions
Chemistry: Atoms First
- It is possible to write a simple Lewis structure for the SO42- ion, involving only single bonds, which follows the octet rule. However, Linus Pauling and others have suggested an alternative structure, involving double bonds, in which the sulfur atom is surrounded by six electron pairs. (a) Draw the two Lewis structures. (b) What geometries are predicted for the two structures? (c) What is the hybridization of sulfur in each case? (d) What are the formal charges of the atoms in the two structures?arrow_forwardIn each of the following molecules, a central atom is surrounded by a total of three atoms or unshared electron pairs: SnCl2, BCl3, SO2. In which of these molecules would you expect the bond angle to be less than 120? Explain your reasoning.arrow_forwardCould the anion Li2 exist? What is the ions bond order?arrow_forward
- Among the following, which has the shortest bond and which has the longest: Li2, B2, C2, N2, O2?arrow_forwardWhich of these molecules have an odd number of valence electrons: NO2, SCl2, NH3, NO3?arrow_forwardWhat are the bond angles predicted by the VSEPR model about the carbon atom in the formate ion, HCO2? Considering that the bonds to this atom are not identical, would you expect the experimental values to agree precisely with the VSEPR values? How might they differ?arrow_forward
- Explain in terms of bonding theory why all four hydrogen atoms of allene, H2CCCH2, cannot lie in the same plane.arrow_forwardA neutral molecule is identified as a tetrafluoride, XF4, where X is an unknown atom. If the molocule has a dipole moment of 0.63 D, can you give some possibilities for the identity of X?arrow_forwardBest Lewis Formula and Molecular Geometry A student writes the Lewis electron-dot formula for the carbonate anion, CO32, as a Does this Lewis formula obey the octet rule? Explain. What are the formal charges on the atoms? Try describing the bonding for this formula in valence bond terms. Do you have any difficulty doing this? b Does this Lewis formula give a reasonable description of the electron structure, or is there a better one? If there is a better Lewis formula, write it down and explain why it is better. c The same student writes the following resonance description for CO2: Is there something wrong with this description? (What would you predict as the geometries of these formulas?) d Is one or the other formula a better description? Could a value for the dipole moment help you decide? e Can you write a Lewis formula that gives an even better description of CO2? Explain your answer.arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





